


Clinical research stands as a systematic investigation of health interventions, meticulously aimed at assessing their safety and efficacy. This endeavor is crucial for advancing medical knowledge and significantly improving patient outcomes. The article underscores the importance of clinical research by detailing its historical evolution, methodological advancements, and ethical considerations. These elements collectively illustrate how they contribute to effective healthcare solutions and enhance the overall understanding of medical treatments.
As we delve deeper into the Medtech landscape, it becomes evident that collaboration is essential in addressing key challenges, paving the way for innovative healthcare strategies.
Clinical research stands as a cornerstone of modern medicine, embodying the systematic investigation of health interventions to enhance patient outcomes. This vital field not only ensures the safety and efficacy of new treatments but also drives the evolution of medical knowledge and practice.
However, as the landscape of clinical research continues to evolve, questions arise about its methodologies, ethical considerations, and the challenges of participant engagement.
How can stakeholders navigate these complexities to harness the full potential of clinical research for better healthcare solutions?
The term clinical research meaning encompasses clinical investigation, which is a vital branch of medical inquiry that systematically examines health interventions such as drugs, medical devices, and treatment protocols to assess their safety and efficacy in human subjects. This area is crucial for enhancing medical understanding and improving health outcomes, as it provides the necessary evidence for regulatory approvals and the establishment of treatment guidelines, which aligns with the clinical research meaning. The conversion of scientific findings into practical healthcare applications is a significant advantage of medical studies, ultimately elevating individual well-being.
Real-world instances underscore the profound impact of medical research on health outcomes. For instance, bioaccess® leverages over 20 years of experience in Medtech to manage various research studies, including:
Their strategic approach facilitates accelerated patient enrollment, achieving cohorts 50% faster than traditional Western sites, while also generating substantial cost savings of $25K per patient with FDA-ready data—no rework, no delays. This efficiency not only streamlines the medical study process but also bolsters local economies through job creation and healthcare advancements.
Furthermore, Walgreens' commitment to integrating women into research studies has resulted in over 4 million potential enrollments, with more than 60% being women. This initiative exemplifies a successful effort to enhance diversity and improve healthcare results.
Expert perspectives highlight the essential role of medical studies, emphasizing the clinical research meaning in healthcare. Leaders in the field emphasize that methodologically robust studies are critical for establishing a solid evidence base that informs the clinical research meaning. Recent advancements in medical study methodologies, such as the emergence of virtual trial platforms and AI-driven analytics, are revolutionizing study conduct, rendering them more efficient and accessible.
Statistics illustrate the significant influence of medical studies on individual outcomes. Despite challenges, such as under-enrollment in trials—where 37% fail to enroll sufficient patients—the clinical research meaning remains a cornerstone for developing effective treatments. The ongoing evolution of medical investigation methodologies enhances the quality of evidence available, ultimately leading to improved healthcare solutions for patients worldwide.
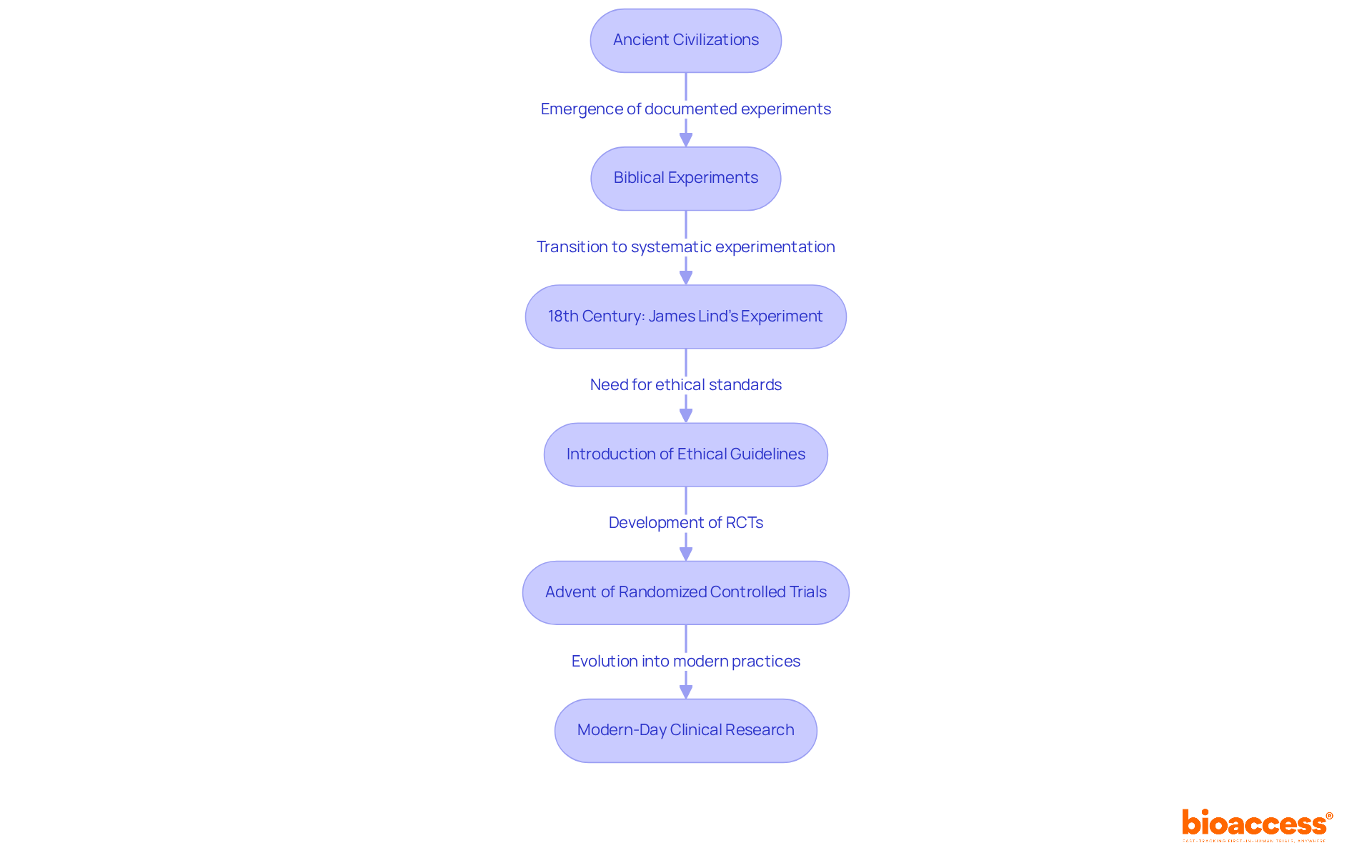
The evolution of medical studies traces back to ancient civilizations, with the earliest documented experiments emerging in biblical times. A notable example is the dietary test conducted by King Nebuchadnezzar, which revealed the health benefits of a vegetarian diet compared to a meat-based one. However, the modern era of medical research began in the 18th century, marked by James Lind's controlled experiment on scurvy in 1747. Lind's approach, which involved dividing sailors into groups to assess the efficacy of citrus fruits, established a pivotal foundation for future medical trials and underscored the importance of systematic experimentation.
Over the years, medical investigations have undergone significant transformations. The introduction of ethical guidelines, particularly the Nuremberg Code and the Declaration of Helsinki, emerged in response to historical violations of human rights in medical studies. These documents emphasize the necessity of informed consent and the protection of human participants, thereby shaping the ethical framework governing research studies.
The advent of randomized controlled trials (RCTs) has further revolutionized medical research, providing a robust framework for evaluating the safety and efficacy of new therapies. By the early 21st century, the global landscape of medical studies expanded remarkably, with over 452,604 registered studies as of May 2023, reflecting an increasing commitment to rigorous scientific inquiry.
Key milestones in this progression include:
These services not only enhance research efficiency but also bolster local economies through job creation, economic growth, and improved healthcare outcomes.
Historical case studies, such as Ambroise Paré's unintentional medical experiment in the 16th century, which led to the discovery of a more effective treatment for wounds, illustrate the ongoing innovation within the field. Today, understanding the clinical research meaning is crucial not only for drug development but also for significantly advancing medical knowledge and improving patient outcomes.
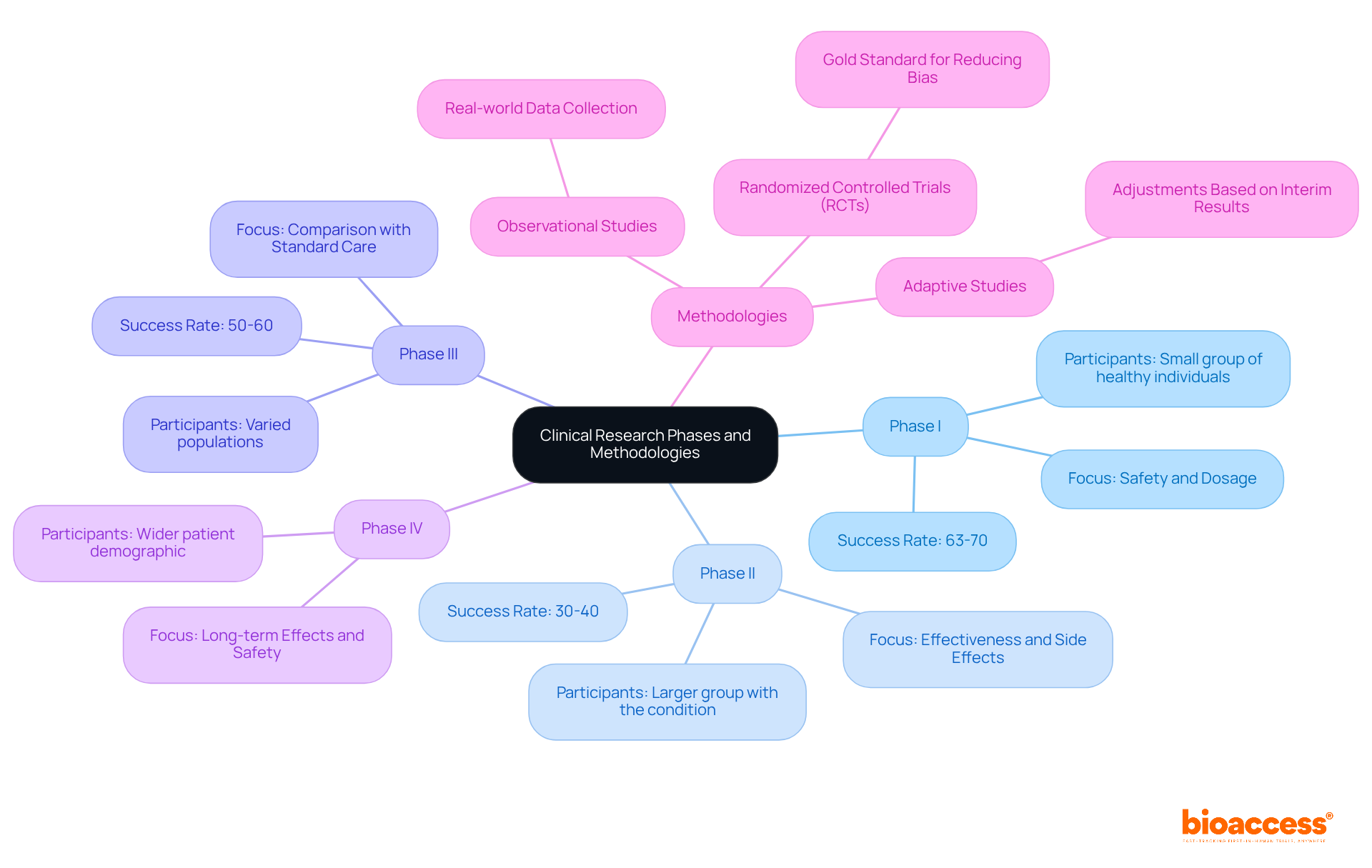
The clinical research meaning involves systematically categorizing the process into four distinct phases, each serving a critical role in the development of new medical interventions.
The methodologies employed in clinical research meaning are diverse and tailored to specific study objectives.
Statistics show the differing success rates across clinical study phases, with roughly 63%-70% of drugs successfully passing Phase I, while only 30%-40% move from Phase II to Phase III. The overall approval rate for new drugs hovers around 10%, highlighting the challenges faced in clinical research meaning related to drug development. Real-world examples demonstrate the use of these methodologies; for instance, recent adaptive studies in oncology have shown promise in identifying effective treatment combinations more efficiently.
In this context, bioaccess® offers accelerated regulatory approval in just 6-8 weeks, significantly faster than the typical 6-12 months seen in the US and EU. This capability enables the enrollment of treatment-naive cardiology or neurology groups at a pace 50% quicker than Western sites, tackling typical recruitment challenges encountered by Medtech and Biopharma startups. Additionally, bioaccess® offers extensive trial management services, including Early-Feasibility Studies, First-In-Human Studies, and Post-Market Follow-Up Studies, ensuring a tailored approach to navigating trials.
Quotes from industry leaders emphasize the significance of these phases: "The effects of Phase III failures are significant, impacting both human lives and financial costs," highlights Sy Pretorius, Chief Scientific Officer at PAREXEL. This highlights the importance of strict methodologies and ethical compliance throughout the medical investigation process, ensuring that new therapies are both safe and effective for individuals, while also contributing to local economies and healthcare advancements.
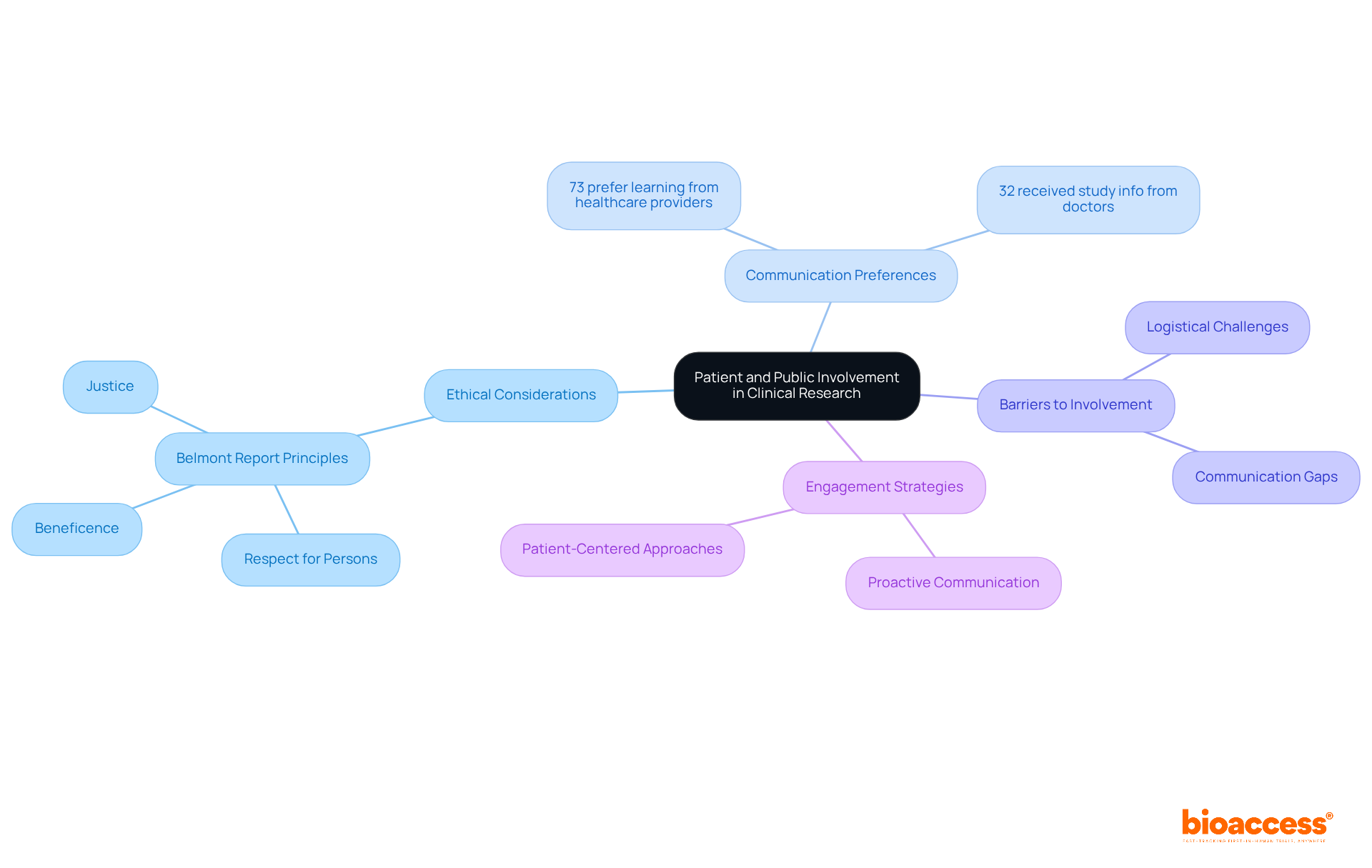
Ethical considerations in clinical studies are paramount, ensuring that the rights, safety, and well-being of participants are protected. Engaging individuals and the community in the investigation process not only builds trust but also enhances the significance of studies. Ethical guidelines, such as the Belmont Report, outline principles like respect for persons, beneficence, and justice, which serve as a compass for researchers in their conduct. Involving individuals as collaborators in research not only improves recruitment and retention but also yields more significant outcomes that align with their needs and preferences.
Data reveals that while 73% of individuals prefer to learn about research study opportunities from their healthcare providers, only 32% indicated that their doctors had ever communicated information about studies with them. This communication gap underscores the necessity for effective strategies to bridge the divide. Furthermore, research indicates that when individuals are educated about research studies by their healthcare professionals, they are considerably more inclined to participate, emphasizing the importance of proactive communication. Notably, merely 5% of research trial participants discovered studies via online support groups, highlighting that traditional communication methods remain essential.
Addressing logistical barriers to involvement is equally crucial, as these challenges can impede individual engagement. Continuous efforts to enhance patient engagement strategies must ensure that ethical considerations remain at the forefront of clinical research meaning.
The exploration of clinical research reveals its pivotal role in advancing medical knowledge and enhancing patient care. By systematically investigating health interventions, clinical research not only provides the evidence needed for regulatory approvals but also translates scientific discoveries into practical applications that significantly improve individual health outcomes. Understanding the core meaning of clinical research is essential for recognizing its impact on healthcare practices and policies.
Key arguments highlighted throughout this discussion include:
From the early experiments of ancient civilizations to modern advancements in research techniques, the journey of clinical research underscores a commitment to improving treatment safety and efficacy. Notably, the integration of diverse populations in studies, as seen in initiatives like Walgreens’ efforts to include women, exemplifies the ongoing evolution toward more inclusive and representative research practices.
The significance of clinical research extends beyond the confines of laboratories and hospitals; it is a vital component of the healthcare ecosystem that shapes the future of medicine. As methodologies continue to advance, and as patient engagement strategies become more refined, the potential for clinical research to drive meaningful change in health outcomes becomes ever more apparent. Embracing this understanding not only fosters a greater appreciation for the research process but also encourages active participation in clinical trials, paving the way for innovative treatments and improved healthcare for all.
What is the core meaning of clinical research?
Clinical research refers to clinical investigation, which systematically examines health interventions like drugs, medical devices, and treatment protocols to assess their safety and efficacy in human subjects.
Why is clinical research important?
Clinical research is crucial for enhancing medical understanding and improving health outcomes. It provides the necessary evidence for regulatory approvals and the establishment of treatment guidelines, ultimately converting scientific findings into practical healthcare applications.
What are some examples of studies managed by bioaccess®?
Bioaccess® manages various research studies, including Early-Feasibility Studies, First-In-Human Studies, and Post-Market Follow-Up Studies.
How does bioaccess® improve patient enrollment in studies?
Bioaccess®'s strategic approach facilitates accelerated patient enrollment, achieving cohorts 50% faster than traditional Western sites, while also generating substantial cost savings of $25K per patient with FDA-ready data.
What initiative has Walgreens undertaken to enhance diversity in research studies?
Walgreens has committed to integrating women into research studies, resulting in over 4 million potential enrollments, with more than 60% being women.
What advancements are impacting clinical research methodologies?
Recent advancements include the emergence of virtual trial platforms and AI-driven analytics, which are revolutionizing study conduct, making them more efficient and accessible.
What challenges does clinical research face regarding patient enrollment?
A significant challenge is under-enrollment in trials, where 37% of studies fail to enroll sufficient patients.
How does clinical research contribute to healthcare solutions?
Clinical research enhances the quality of evidence available, leading to the development of effective treatments and improved healthcare solutions for patients worldwide.