


Clinical trial management is crucial for the oversight of clinical research projects, ensuring their execution is efficient, ethical, and adheres to regulatory standards. This article underscores that effective management safeguards participant welfare and data integrity while simultaneously enhancing recruitment and retention rates. Such improvements lead to better patient outcomes and successful study results, establishing the foundation for future advancements in the Medtech landscape.
Collaboration among stakeholders is essential to address the key challenges faced in clinical research, paving the way for innovative solutions. Ultimately, the importance of robust clinical trial management cannot be overstated; it is a pivotal element that drives the success of clinical research initiatives.
Clinical trial management stands as the backbone of clinical research, ensuring that studies are executed with precision, ethical integrity, and regulatory compliance. This critical oversight not only safeguards participant welfare but also enhances data reliability, paving the way for meaningful medical advancements.
However, with approximately 80% of clinical studies facing delays due to recruitment challenges, one must consider:
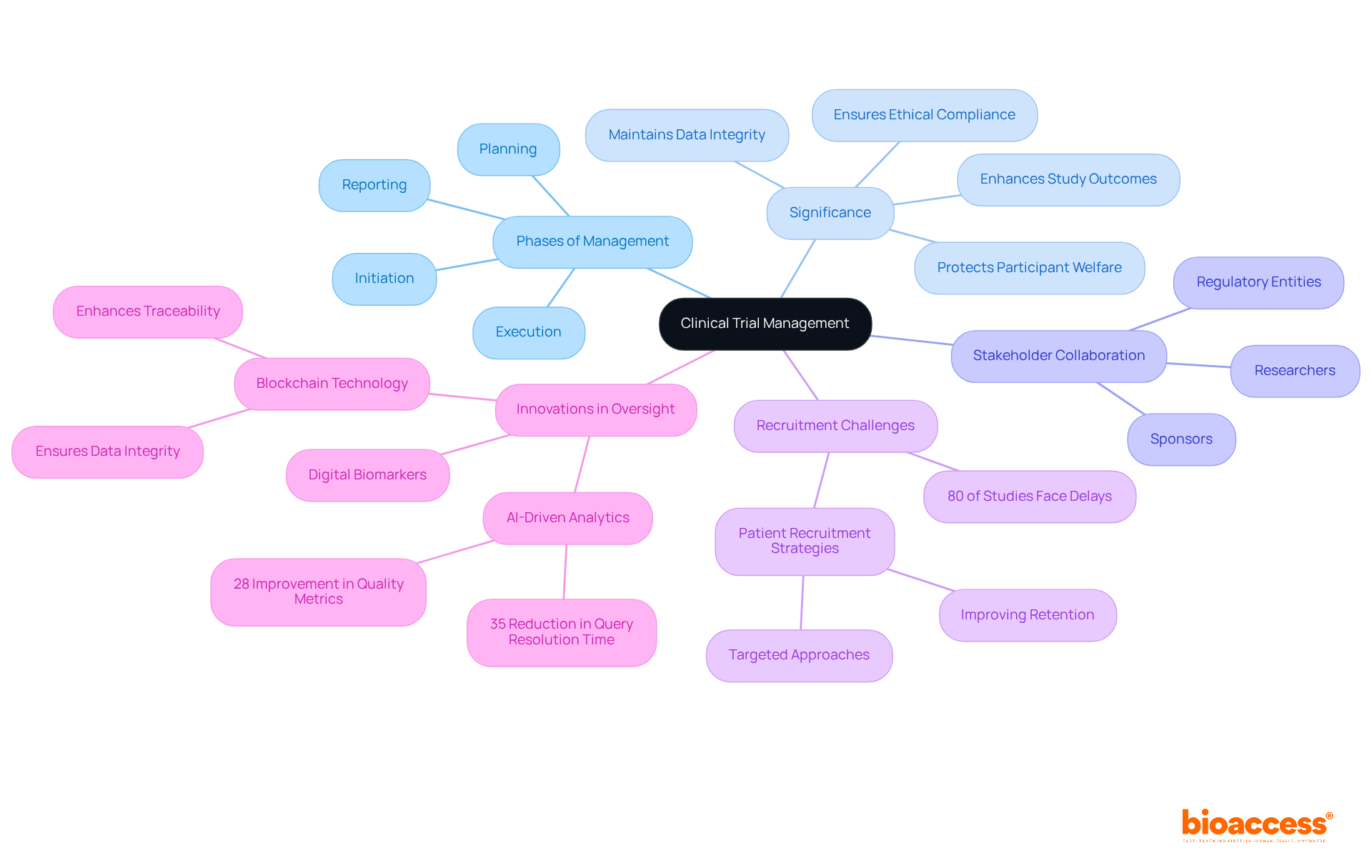
Clinical trial management represents a critical component in the oversight of clinical research projects, guiding them through every phase from planning and initiation to execution and reporting. Its significance lies in ensuring that experiments are conducted efficiently, ethically, and in strict compliance with regulatory standards. Effective oversight in clinical trial management not only protects participant welfare but also ensures data integrity, ultimately leading to reliable results. This process necessitates collaboration among various stakeholders, including researchers, sponsors, and regulatory entities, to facilitate seamless clinical trial management and ensure the timely completion of studies.
The importance of clinical study oversight is underscored by the fact that approximately 80% of clinical studies face delays or terminations due to recruitment challenges, highlighting the pressing need for strategic supervision. Notable examples in the Medtech sector demonstrate how robust administrative practices can enhance patient recruitment and retention, thereby improving study outcomes. For instance, a biotech company specializing in rare diseases achieved a 65% increase in patient recruitment through targeted strategies, showcasing the profound impact of effective oversight on study success.
Moreover, the oversight provided by clinical trial management is pivotal in ensuring ethical compliance and data integrity. The integration of advanced information management solutions, such as AI-driven analytics, has been shown to improve quality metrics by 28% and reduce query resolution time by 35%. These innovations not only bolster the reliability of research data but also foster trust among stakeholders, which is essential for the successful execution of medical studies.
In conclusion, overseeing research studies is indispensable for advancing medical inquiry, as it directly influences the effectiveness, ethical standards, and integrity of research activities. This, in turn, facilitates the development of innovative treatments that enhance patient care.
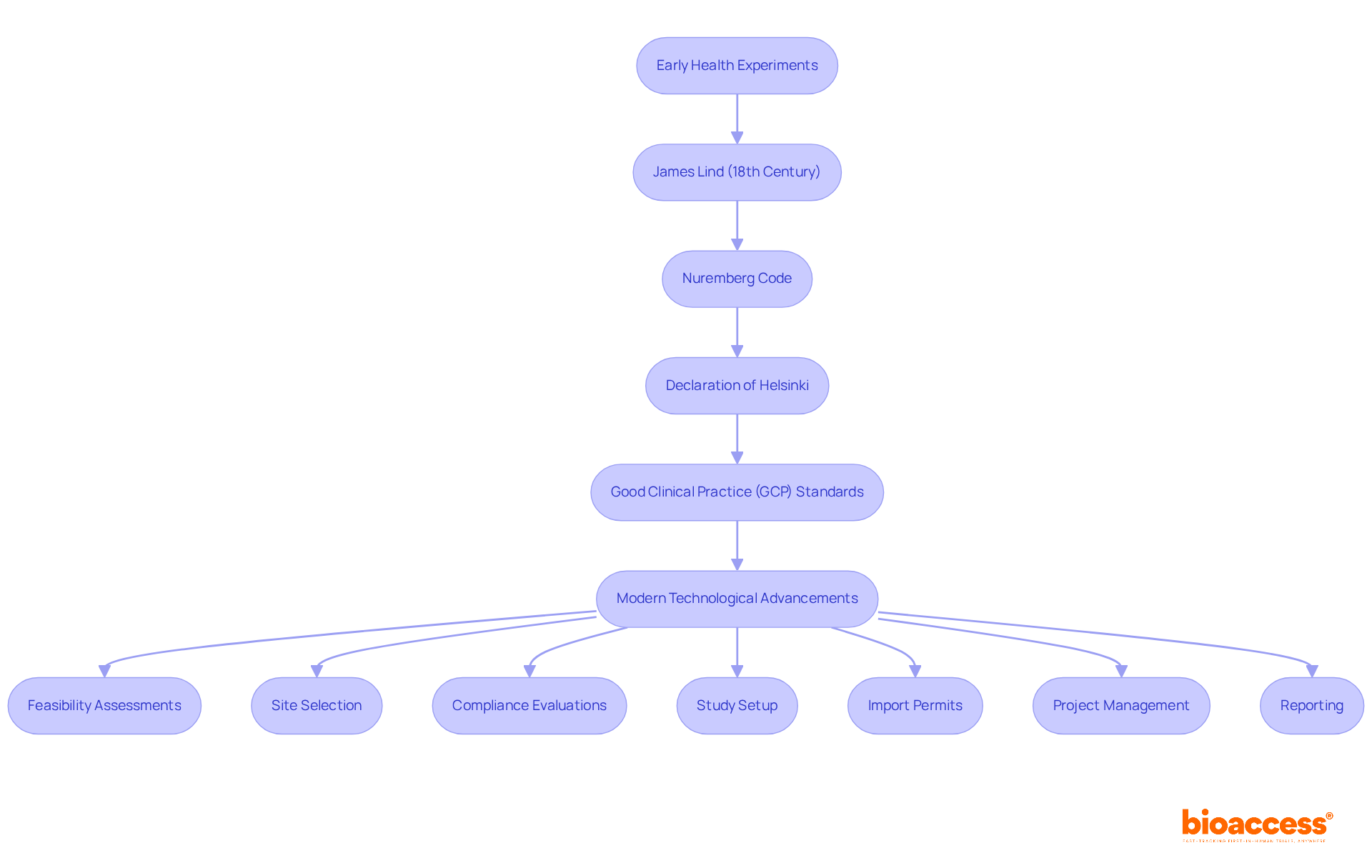
The evolution of medical study oversight can be traced back to early health experiments, notably those conducted by James Lind in the 18th century. This field has undergone significant transformation over the years, particularly following pivotal regulatory milestones such as the Nuremberg Code and the Declaration of Helsinki, which established ethical guidelines for human research.
The Nuremberg Code, introduced in response to the atrocities of World War II, set forth principles that continue to shape ethical standards in research today, emphasizing the necessity of voluntary participation and the right to withdraw. Following these developments, the introduction of Good Clinical Practice (GCP) standards further refined clinical trial management practices, highlighting the need for rigorous oversight and participant protection.
Statistics indicate that compliance with GCP standards has led to enhanced study integrity and participant safety, fostering greater trust in medical research. For instance, the MRC Patulin Clinical Trials Committee's study highlighted the significance of blinding in research studies, illustrating the progression of regulatory practices and their practical consequences.
Today, advancements in technology, such as electronic data capture and clinical trial management, have revolutionized study conduct, enabling more efficient data processing and compliance tracking. In this context, bioaccess offers extensive clinical trial management services, including:
These services are vital for ensuring that medical studies are conducted effectively and in accordance with regulatory standards. As digital health evolves from a curiosity to a widely utilized resource, these innovations enhance effective communication among stakeholders, ultimately resulting in more efficient and transparent research studies.
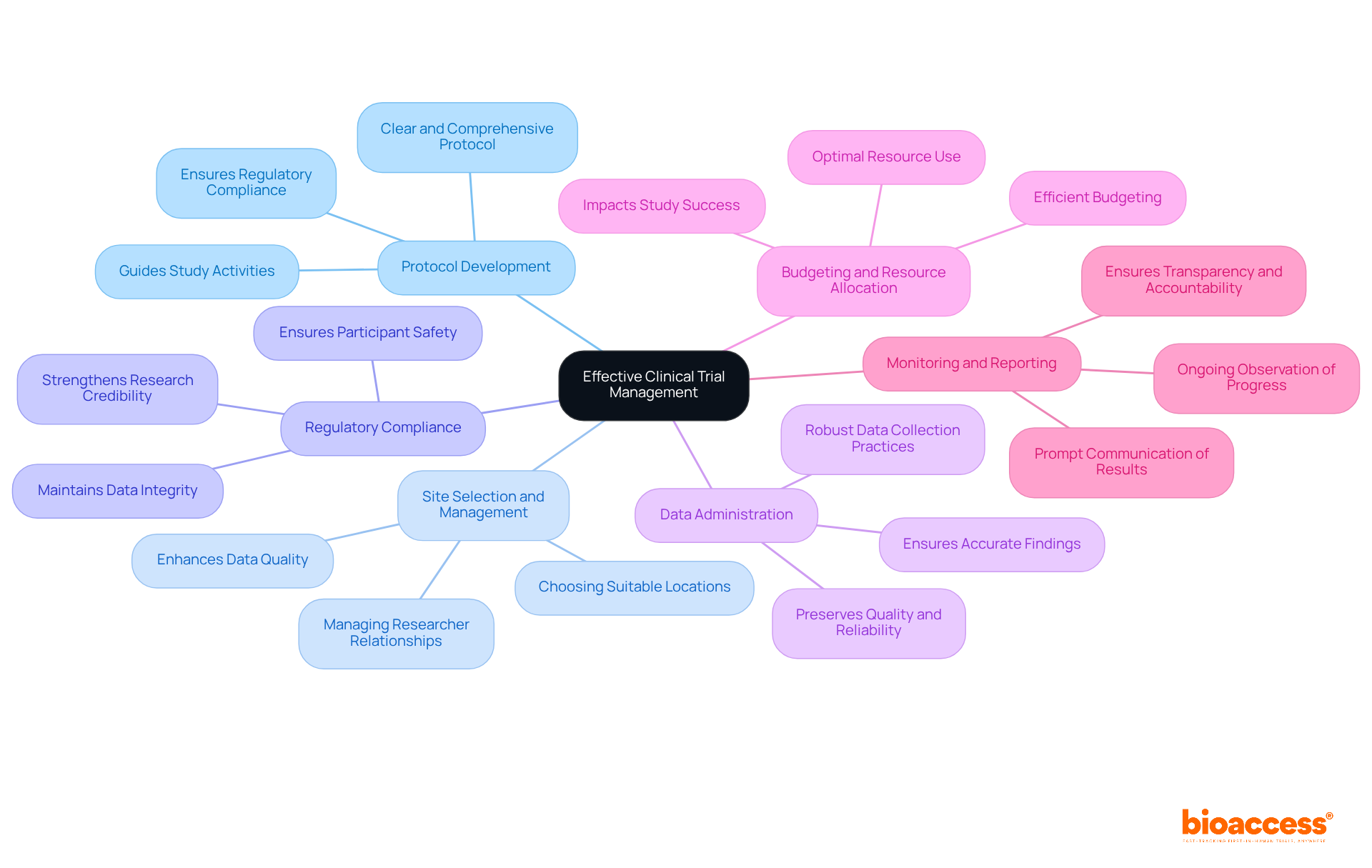
Key components of effective clinical trial management are essential for the success of any clinical research initiative.
Protocol Development: Crafting a clear and comprehensive trial protocol is essential for guiding the study and ensuring compliance with regulatory requirements. This foundational step lays the groundwork for all subsequent activities.
Site Selection and Management: Choosing suitable locations and managing relationships with researchers and site personnel is crucial for recruitment and information gathering. Effective clinical trial management fosters collaboration and enhances data quality.
Adhering to local and international regulations is paramount in clinical trial management, as it ensures participant safety and the integrity of the information collected. Compliance not only protects participants but also strengthens the credibility of the research.
Data Administration: Implementing robust data collection and clinical trial management practices is essential for preserving the quality and reliability of test results. Strong data management practices ensure that findings are both accurate and actionable.
Efficient budgeting and resource allocation in clinical trial management are critical to guarantee that tests are finalized promptly and within budget. Strategic budgeting enables the optimal use of resources, directly impacting the study's success.
Monitoring and reporting are essential components of clinical trial management, as ongoing observation of progress and prompt communication of results are crucial for ensuring transparency and accountability. Continuous monitoring allows for timely adjustments and reinforces stakeholder confidence in the research process.
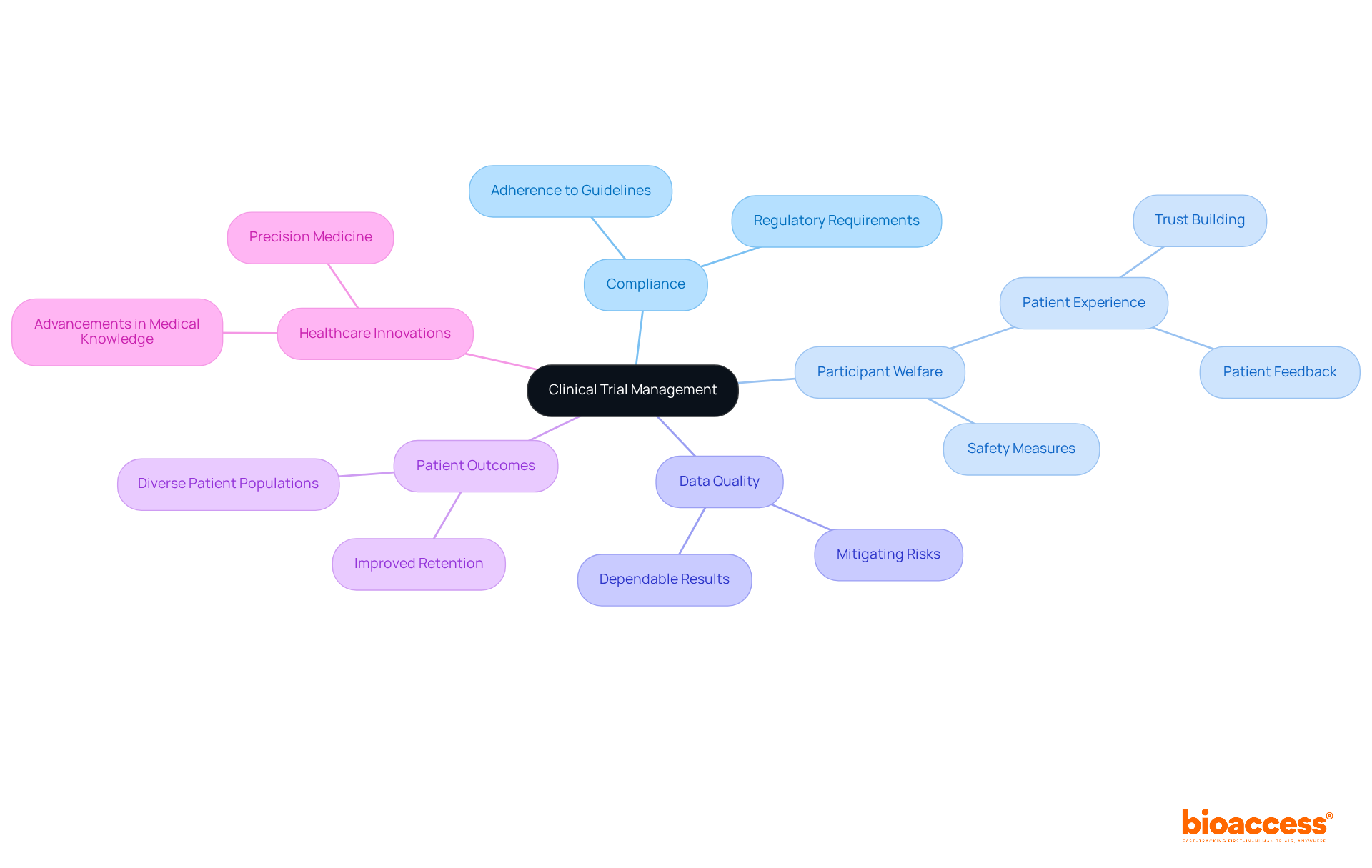
Effective clinical trial management is paramount for ensuring compliance with regulatory requirements, which safeguard participant welfare and uphold the integrity of research. By adhering strictly to established guidelines, study managers can significantly mitigate risks associated with participant safety and data quality.
Well-conducted studies not only enhance the quality of care and monitoring provided to participants but also lead to improved patient outcomes. Research indicates that experiments characterized by strong management practices can enhance patient retention and satisfaction, ultimately fostering trust among stakeholders. This trust is essential, as it bolsters the credibility of the research and supports successful study results, paving the way for advancements in medical knowledge.
Furthermore, adherence in research studies correlates with increased success rates; studies that comply with regulatory standards are more likely to yield dependable results, which can translate into effective treatments and therapies for patients. Statistics reveal that well-managed studies can diminish the likelihood of under-enrollment, a common issue affecting 37% of research studies, thereby ensuring diverse patient populations are represented and that findings are applicable to broader demographics.
Overall, the impact of effective clinical trial management goes beyond mere regulatory compliance, directly influencing patient outcomes and the advancement of healthcare innovations.
Clinical trial management stands as the cornerstone of clinical research, ensuring that studies are executed with precision, ethical integrity, and regulatory compliance. Its role is paramount in safeguarding participant welfare while upholding data integrity, which ultimately leads to trustworthy results. The collaborative nature of clinical trial management, involving researchers, sponsors, and regulatory bodies, is essential for effectively navigating the complexities of clinical studies.
This article underscores several key components that contribute to successful clinical trial management:
Each element is crucial in enhancing study outcomes, as evidenced by statistics demonstrating improved patient recruitment and retention rates when effective management practices are employed. Moreover, the historical evolution of clinical trial management practices highlights the significance of ethical guidelines and technological advancements in shaping modern research methodologies.
Reflecting on the significance of clinical trial management reveals that its impact extends beyond mere compliance and regulatory adherence. Effective management not only enhances patient outcomes and satisfaction but also fosters trust among stakeholders, paving the way for groundbreaking medical innovations. As the landscape of clinical research continues to evolve, prioritizing robust clinical trial management practices will remain essential for advancing healthcare and delivering effective treatments to diverse patient populations.
What is clinical trial management?
Clinical trial management refers to the oversight of clinical research projects, guiding them through all phases from planning and initiation to execution and reporting, ensuring efficiency, ethical conduct, and compliance with regulatory standards.
Why is clinical trial management important?
It is crucial because it protects participant welfare, ensures data integrity, and leads to reliable results. Effective management also addresses recruitment challenges, which can lead to delays or terminations of clinical studies.
What challenges do clinical studies face regarding recruitment?
Approximately 80% of clinical studies experience delays or terminations due to recruitment challenges, highlighting the need for strategic oversight in clinical trial management.
Can you provide an example of successful patient recruitment in clinical trials?
A biotech company specializing in rare diseases achieved a 65% increase in patient recruitment through targeted strategies, demonstrating the positive impact of effective oversight on study outcomes.
How does clinical trial management ensure ethical compliance and data integrity?
Clinical trial management integrates advanced information management solutions, such as AI-driven analytics, which improve quality metrics and reduce query resolution time, thereby bolstering the reliability of research data and fostering trust among stakeholders.
What is the overall impact of clinical trial management on medical research?
Effective oversight in clinical trial management is essential for advancing medical inquiry, influencing the effectiveness, ethical standards, and integrity of research activities, which facilitates the development of innovative treatments that enhance patient care.