


The CPNP, or Cosmetic Product Notification Portal, serves as a crucial online platform established by the European Commission, facilitating the notification and regulatory compliance of beauty products intended for the European market. This system significantly enhances consumer protection by ensuring that all cosmetic items are meticulously reported and evaluated for safety prior to reaching consumers. Such rigorous oversight not only promotes effective regulatory compliance but also upholds high-quality standards within the beauty industry.
The beauty industry is experiencing a profound transformation, propelled by heightened consumer awareness and rigorous regulatory standards. At the heart of this evolution lies the Cosmetic Product Notification Portal (CPNP), an essential tool that guarantees compliance and safety for cosmetic products throughout Europe. As manufacturers navigate the intricate landscape of regulatory requirements, the CPNP presents both a challenge and an opportunity.
How can businesses effectively harness this platform to not only meet compliance standards but also bolster consumer trust in their products?
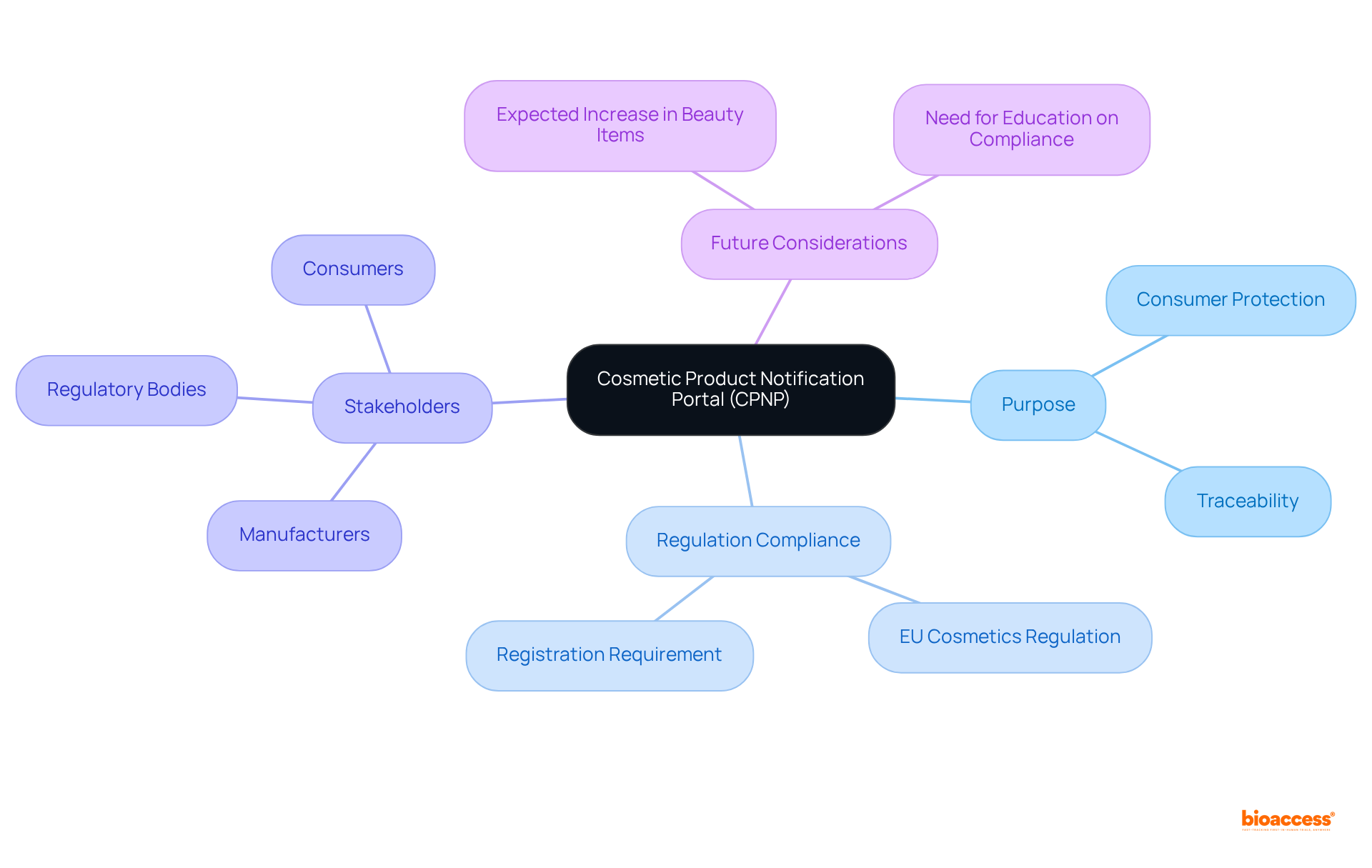
The Cosmetic Item Notification Portal (CINP) serves as a crucial online platform established by the European Commission for the notification of beauty items intended for the European market. This centralized database mandates that manufacturers and importers provide comprehensive information about their beauty items prior to marketing. The portal is instrumental in enhancing consumer protection and ensuring compliance with the EU Cosmetics Regulation (EC) No 1223/2009, which stipulates that all beauty items must be registered in this system to facilitate traceability and regulatory oversight.
By streamlining the notification process, the organization fosters effective communication among stakeholders, including producers, regulatory bodies, and consumers, thereby underscoring the importance of compliance in maintaining high-quality standards within the beauty industry. As Paul Koziarz points out, businesses must continually educate themselves about compliance and security to keep pace with evolving regulations and sophisticated cyber threats.
Furthermore, with the anticipated rise in the number of beauty items in 2025, it is imperative for manufacturers to leverage this platform effectively to ensure their products meet regulatory standards and consumer protection guidelines.
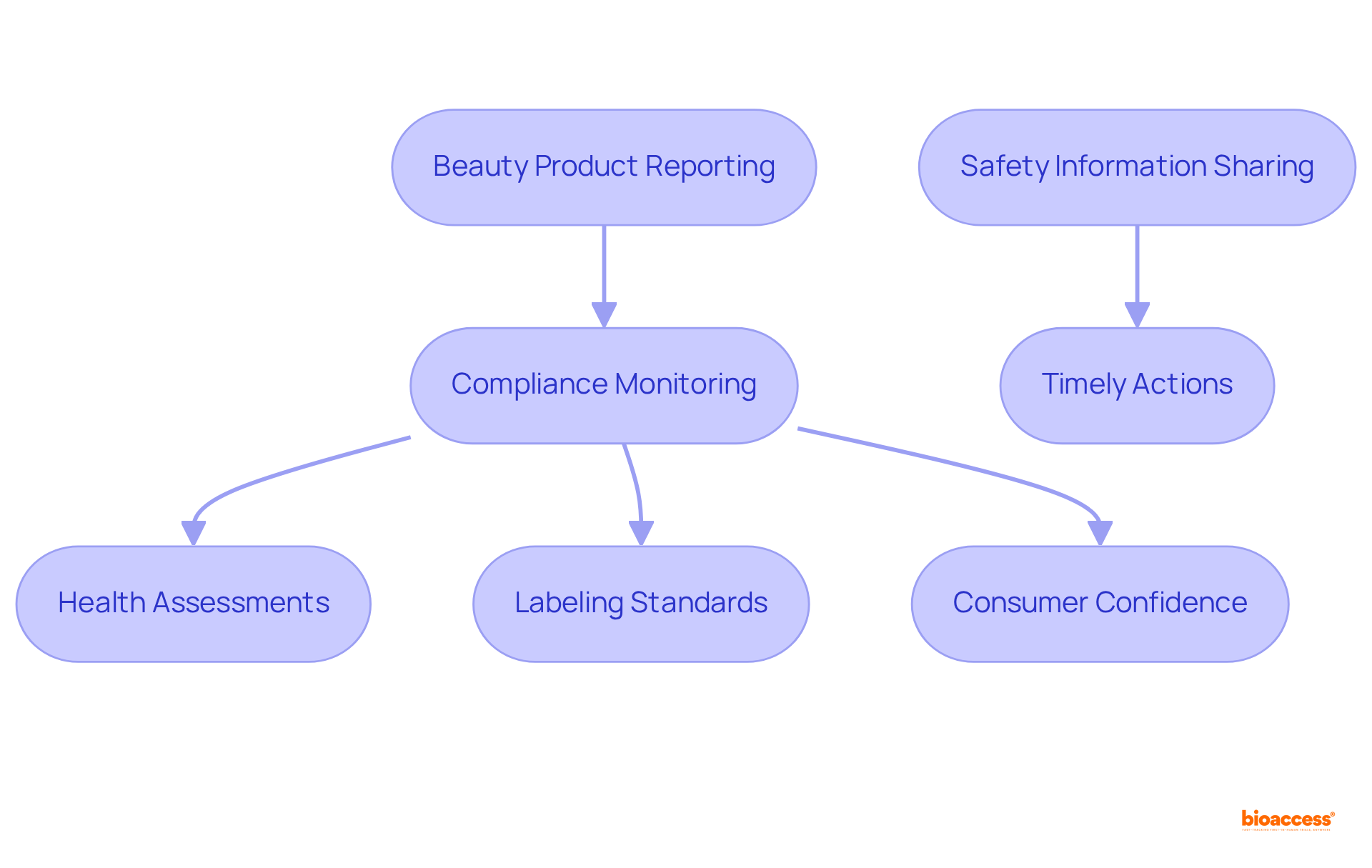
The organization plays a crucial role in ensuring that beauty products comply with health regulations before they reach consumers. By mandating that all beauty items be reported through this portal, the EU aims to protect public health and well-being. This framework empowers regulatory authorities to monitor products available in the market, ensuring adherence to health assessments and labeling standards. Such regulatory compliance is vital for maintaining consumer confidence and preventing adverse health effects associated with unsafe beauty products. Furthermore, the network facilitates swift sharing of safety information, enabling timely actions in the event of recalls or safety alerts.
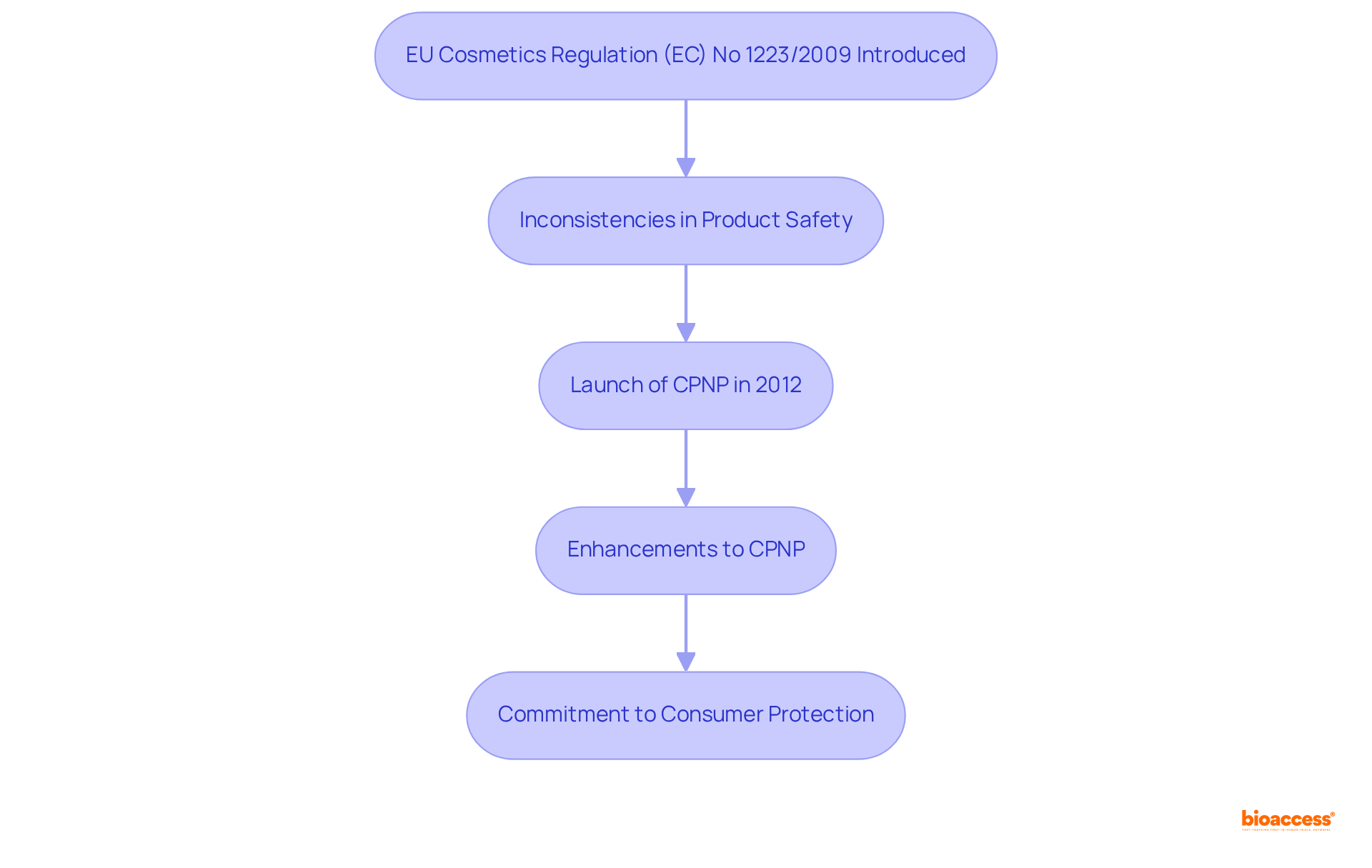
The establishment of the CPNP meaning was a pivotal component of the EU's initiative to standardize beauty product regulations across its member states. The introduction of the EU Cosmetics Regulation (EC) No 1223/2009 represented a transformative shift in the regulation of cosmetic products in Europe. Prior to this regulation, the absence of a uniform notification system led to inconsistencies in product safety and compliance. In response to these challenges, the program was launched in 2012, providing a centralized platform for notifications. Over the years, the program has undergone enhancements aimed at improving its functionalities and adapting to emerging concerns, underscoring the EU's commitment to consumer protection and regulatory efficacy.
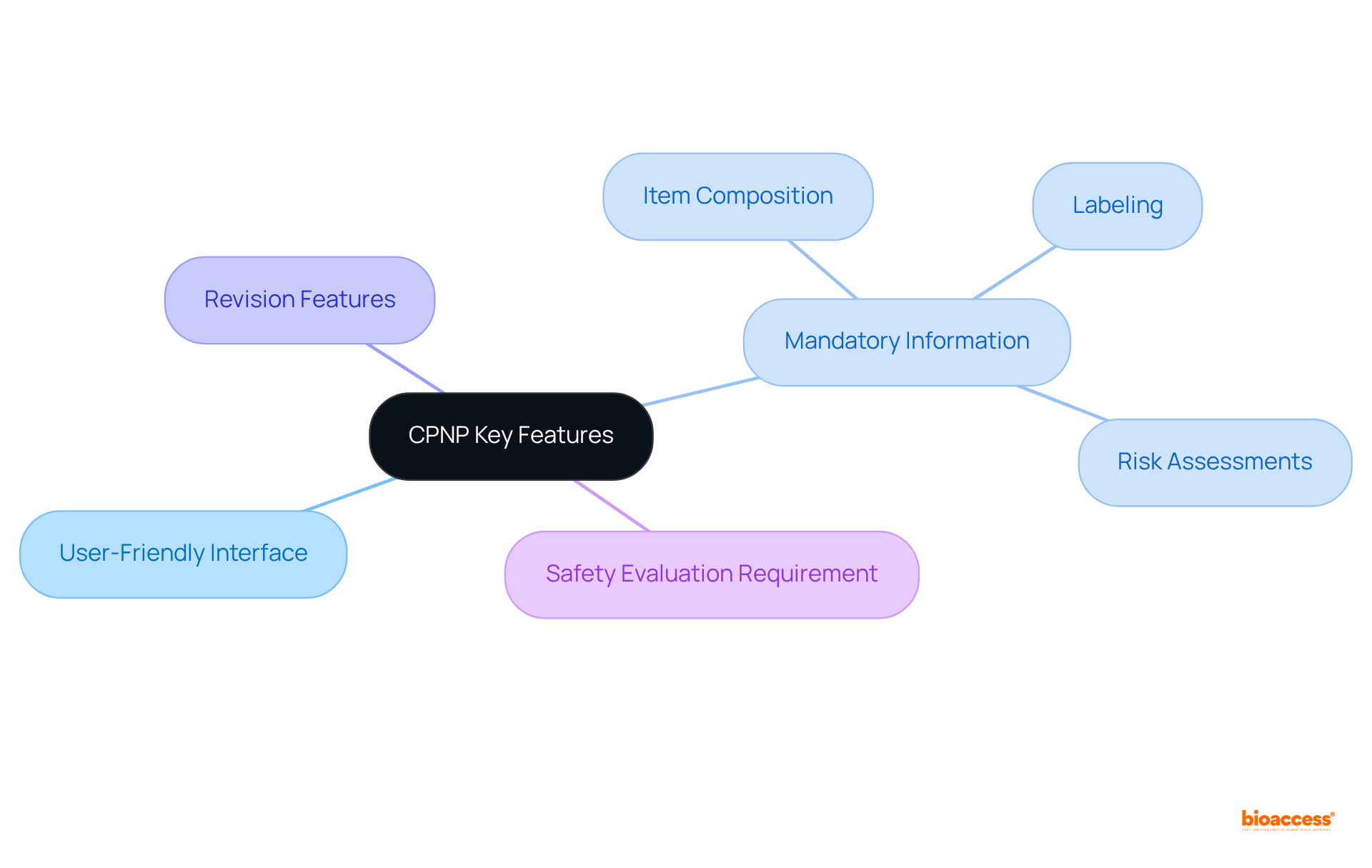
The program's key attributes include a user-friendly interface that allows manufacturers to seamlessly submit notifications regarding their items. The portal mandates specific information, such as item composition, labeling, and risk assessments, thereby ensuring that all essential data is gathered for regulatory review. Additionally, the network provides features for revising item details and notifying authorities of any changes. A critical prerequisite for utilizing this system is that all cosmetic items must undergo a safety evaluation conducted by a qualified expert prior to notification. This requirement guarantees that only safe products reach consumers, thereby reinforcing the commitment of the CPNP meaning to promoting public health.
The Cosmetic Product Notification Portal (CPNP) is pivotal in regulating beauty products within the European market. By mandating manufacturers and importers to provide comprehensive information about their products, CPNP not only guarantees adherence to EU regulations but also bolsters consumer safety and trust. This centralized platform is essential for upholding high-quality standards in the beauty industry, as it enables effective communication among stakeholders and empowers regulatory authorities to oversee product safety.
Key points throughout the article underscore the significance of CPNP in advancing public health and regulatory compliance. The platform's user-friendly interface, stringent safety evaluation requirements, and capabilities for prompt updates highlight its crucial role in preventing unsafe products from reaching consumers. Furthermore, the historical context of CPNP's development illustrates its evolution in response to the increasing complexities of the cosmetics industry and the necessity for standardized regulations.
Given the projected growth in beauty products, it is imperative for manufacturers to fully utilize the CPNP to ensure their offerings align with regulatory standards and consumer protection guidelines. As the beauty industry continues to evolve, embracing compliance and safety measures through platforms like CPNP will be vital in fostering consumer confidence and safeguarding public health. Engaging with this system transcends mere regulatory obligation; it embodies a commitment to quality and safety that ultimately benefits both businesses and consumers alike.
What is the Cosmetic Product Notification Portal (CPNP)?
The Cosmetic Product Notification Portal (CPNP) is an online platform established by the European Commission for the notification of beauty items intended for the European market.
What is the purpose of the CPNP?
The CPNP serves to enhance consumer protection and ensure compliance with the EU Cosmetics Regulation (EC) No 1223/2009 by requiring manufacturers and importers to provide comprehensive information about their beauty items prior to marketing.
Why is registration in the CPNP important?
Registration in the CPNP is important for facilitating traceability and regulatory oversight of beauty items, ensuring that they meet high-quality standards and comply with safety regulations.
How does the CPNP benefit stakeholders in the beauty industry?
The CPNP fosters effective communication among stakeholders, including producers, regulatory bodies, and consumers, which helps maintain compliance and high-quality standards within the beauty industry.
What challenges do businesses face regarding compliance with the CPNP?
Businesses must continually educate themselves about compliance and security to keep pace with evolving regulations and sophisticated cyber threats, as highlighted by industry expert Paul Koziarz.
What is expected regarding the number of beauty items in 2025?
There is an anticipated rise in the number of beauty items in 2025, making it imperative for manufacturers to leverage the CPNP effectively to meet regulatory standards and consumer protection guidelines.