


The complex responsibilities within Contract Research Organizations (CROs) are crucial to the success of clinical research, especially in cross-sector programs where various stakeholders come together. By establishing a clear accountability structure, CROs not only ensure compliance with regulatory standards but also promote collaboration that enhances research outcomes.
As the complexity of clinical trials increases, organizations must consider: how can they effectively navigate the challenges of accountability to uphold integrity and transparency?
This article explores the essential components of CRO accountability structures, examining their historical evolution and the significant impact they have on program success.
The responsibility framework of a Contract Research Organization (CRO) is a vital structure that delineates the roles, duties, and expectations of all parties involved in research activities. This framework is essential for ensuring compliance with regulatory guidelines while delivering high-quality research outcomes. Key stakeholders typically include:
Each with specific responsibilities that contribute to the success of research trials.
A robust oversight framework not only fosters transparency and enhances communication but also mitigates risks associated with clinical research. For instance, organizations that prioritize responsibility training for managers cultivate environments where employees feel empowered to innovate and take risks, ultimately leading to improved patient outcomes. Research indicates that teams with well-defined purpose statements achieve performance metrics that are 40% better than those lacking clear direction, underscoring the importance of responsibility in achieving desired results.
Industry leaders assert that responsibility is crucial for effective leadership and driving business success. As Pat Summitt aptly stated, "Responsibility equals answerability equals ownership," highlighting the profound impact of ownership on team dynamics. Furthermore, a study revealed that organizations with strong responsibility cultures enjoy 21% higher profitability and 17% greater productivity than their counterparts, illustrating the tangible advantages of responsibility in CRO operations.
In the realm of clinical trials, responsibility transcends mere procedural requirements; it serves as a foundational element that ensures ethical execution and aligns with both personal and organizational goals. By implementing a CRO accountability structure for cross-sector programs, clear responsibility structures can be established, allowing CROs to enhance their operational efficiency and ultimately yield better outcomes for patients and stakeholders alike.

The responsibility of a Contract Research Organization (CRO) is particularly crucial in establishing a CRO accountability structure for cross-sector programs, where diverse participants collaborate to achieve common goals. In these settings, the CRO accountability structure for cross-sector programs ensures a clear delineation of responsibilities, which helps all parties comprehend their roles and obligations, vital for adhering to regulatory standards and ethical guidelines. This responsibility is underscored by the need for transparency in reporting and decision-making processes, fostering trust among stakeholders.
For instance, consider a research study involving a CRO like bioaccess, a pharmaceutical firm, and regulatory bodies. Here, oversight measures are in place to guarantee data integrity, ensuring that all parties are kept informed about progress and challenges. Notably, bioaccess expedites regulatory approval processes, achieving results in just 6-8 weeks, a stark contrast to the typical 6-12 months in the US and EU. This collaborative approach not only enhances the quality of research but also accelerates the pathway to market for innovative treatments.
A prime example of this is bioaccess's partnership with Caribbean Health Group, which has positioned Barranquilla as a leading hub for medical studies in Latin America. This collaboration has significantly reduced recruitment times by over 50% and achieved impressive retention rates of 95%. Such outcomes highlight the transformative potential of effective collaboration in clinical research.

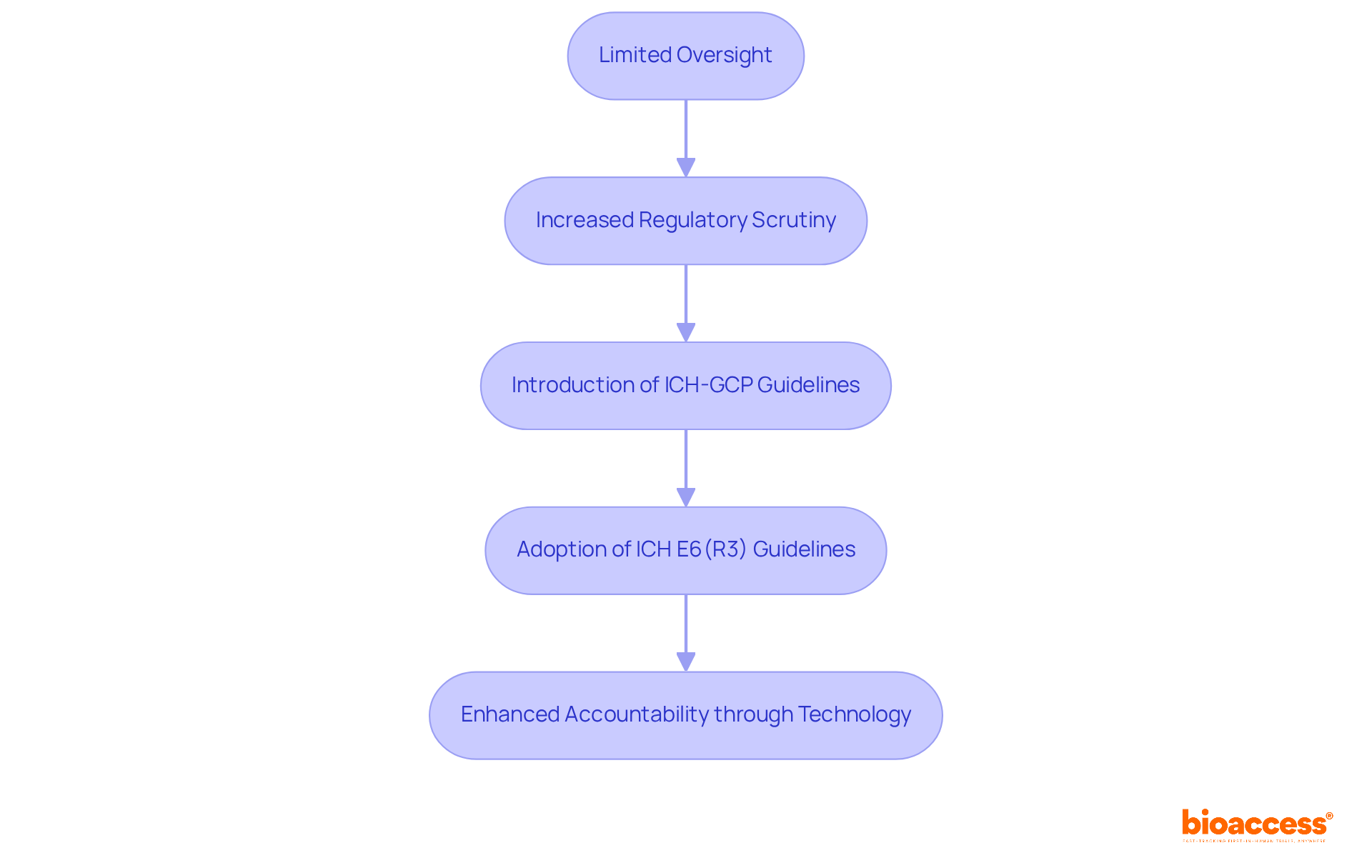
The oversight frameworks of Contract Research Organizations (CROs) have undergone significant transformations in recent decades, primarily driven by evolving regulatory demands, technological advancements, and the increasing complexity of clinical trials. In the early days, CROs operated with limited oversight, often leading to inconsistencies in data quality and compliance. However, as the industry faced heightened scrutiny from regulatory bodies and stakeholders, the necessity for robust oversight frameworks became evident. The introduction of guidelines like ICH-GCP (International Council for Harmonisation - Good Clinical Practice) established a crucial foundation for responsibility, underscoring the importance of clearly defined roles and duties among all parties involved.
As we approach late 2024, the adoption of the revised ICH E6(R3) guidelines, effective July 2025, signifies a pivotal shift towards a principles-based framework that prioritizes proactive quality management and participant safety. This evolution is supported by compelling statistics indicating that modern Phase III studies now involve approximately 263 procedures per patient, reflecting the growing complexity and data demands of contemporary research. Furthermore, the integration of advanced technologies has significantly enhanced accountability, enabling real-time observation and reporting of study data. This technological advancement not only boosts transparency but also fosters trust among stakeholders, as demonstrated by case studies showcasing improved enrollment and cost reductions through decentralized research models during the COVID-19 pandemic.
At bioaccess, we excel in comprehensive trial management services, encompassing feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting. Our expertise ensures effective navigation of the regulatory landscape, providing tailored solutions for Medtech startups aiming for expedited research outcomes. Industry specialists emphasize that responsibility is not merely a regulatory requirement but a fundamental aspect of ethical medical research. As organizations adapt to these evolving standards, the focus on the CRO accountability structure for cross-sector programs will continue to shape the clinical trial environment, ensuring that participant welfare and data integrity remain paramount.
The implementation of a CRO accountability structure for cross-sector programs is crucial in enhancing functionality and reliability within clinical research. At the forefront of these frameworks are:
Clarity ensures that all stakeholders understand their specific duties, significantly reducing misunderstandings and errors. Strong communication channels facilitate timely information sharing, essential for addressing issues as they arise. Notably, statistics reveal that organizations with effective communication practices are 50% more likely to achieve project success, highlighting the critical role of clear dialogue in CRO operations.
Moreover, implementing performance metrics enables the assessment of the CRO accountability structure for cross-sector programs and ensures compliance with regulatory standards. Regular audits and feedback systems can identify areas for improvement, ensuring that the CRO accountability structure for cross-sector programs remains a priority throughout the research process. For example, bioaccess enhances its responsibility framework through comprehensive clinical trial management services, including:
This organized approach has led to improved participant satisfaction and a reduction in project delays.
Furthermore, fostering a culture of responsibility within the CRO and among stakeholders promotes proactive engagement and collaboration. As Patrick Lencioni aptly stated, "trust cannot exist without responsibility," underscoring that this cultural shift not only enhances communication but also leads to superior research outcomes. Teams that prioritize accountability, bolstered by the services provided by bioaccess, are more likely to achieve their objectives and deliver high-quality results.

The CRO accountability structure stands as a cornerstone in the realm of clinical research, ensuring that all stakeholders - from sponsors to regulatory agencies - are aligned in their roles and responsibilities. This framework transcends mere guidelines; it is a vital component that enhances the quality and efficiency of research outcomes, ultimately benefiting patients and the broader healthcare community.
Key arguments throughout this article underscore the importance of clarity in roles, robust communication, and established performance metrics within CRO accountability structures. By fostering a culture of responsibility, organizations can enhance collaboration, mitigate risks, and achieve superior results. The historical evolution of these frameworks reveals a clear trend toward increased oversight and ethical execution, which is essential in today’s complex clinical trial environment.
In summary, the significance of implementing an effective CRO accountability structure cannot be overstated. As the industry evolves, stakeholders must prioritize transparency and responsibility to ensure the success of cross-sector programs. Embracing these principles not only boosts operational efficiency but also reinforces the commitment to ethical research practices that ultimately lead to better patient outcomes. The call to action is unmistakable: invest in accountability to drive innovation and success in clinical research.
What is the CRO accountability structure?
The CRO accountability structure is a framework that defines the roles, duties, and expectations of all parties involved in research activities within a Contract Research Organization (CRO). It ensures compliance with regulatory guidelines and contributes to high-quality research outcomes.
Who are the key stakeholders in a CRO accountability structure?
The key stakeholders typically include sponsors, regulatory agencies, and the CRO itself, each with specific responsibilities that contribute to the success of research trials.
Why is a robust oversight framework important in clinical research?
A robust oversight framework fosters transparency, enhances communication, and mitigates risks associated with clinical research, ultimately leading to improved patient outcomes.
How does responsibility training for managers impact CRO environments?
Organizations that prioritize responsibility training for managers create environments where employees feel empowered to innovate and take risks, which can lead to better patient outcomes.
What is the significance of having well-defined purpose statements in teams?
Research indicates that teams with well-defined purpose statements achieve performance metrics that are 40% better than those lacking clear direction, highlighting the importance of responsibility in achieving desired results.
What are the benefits of a strong responsibility culture in organizations?
Organizations with strong responsibility cultures enjoy 21% higher profitability and 17% greater productivity compared to their counterparts, demonstrating the tangible advantages of responsibility in CRO operations.
How does responsibility relate to ethical execution in clinical trials?
Responsibility serves as a foundational element that ensures ethical execution of clinical trials and aligns with both personal and organizational goals.
What is the impact of implementing a CRO accountability structure for cross-sector programs?
Implementing a CRO accountability structure allows for clear responsibility structures to be established, enhancing operational efficiency and yielding better outcomes for patients and stakeholders.