


The article examines the substantial impact of FDA shutdowns on clinical research operations, underscoring notable delays in medication approvals, inspections, and the overall efficiency of the FDA. This analysis is bolstered by detailing how furloughs of FDA staff during shutdowns create backlogs and compliance challenges. Consequently, these issues restrict patient access to new therapies and complicate the clinical research landscape.
Understanding these dynamics is crucial for stakeholders aiming to navigate the complexities of Medtech and bioaccess, as they play a pivotal role in addressing these key challenges. The importance of collaboration in overcoming these obstacles cannot be overstated, as it lays the groundwork for more efficient and effective clinical research processes.
The Food and Drug Administration (FDA) plays a crucial role in safeguarding public health through the regulation of food, medications, and medical devices. However, government shutdowns significantly disrupt the FDA's operations, creating a ripple effect throughout the clinical research landscape. This article explores the profound implications of FDA shutdowns, focusing on how they:
As stakeholders navigate these turbulent waters, a pressing question arises: how can clinical research teams adapt to ensure compliance and maintain momentum in the face of such uncertainty?
The Food and Drug Administration (FDA) is pivotal in overseeing food, medications, and medical devices in the United States. However, during an FDA shutdown, its operations can face significant interruptions.
For instance, during the FDA shutdown of 2018-2019:
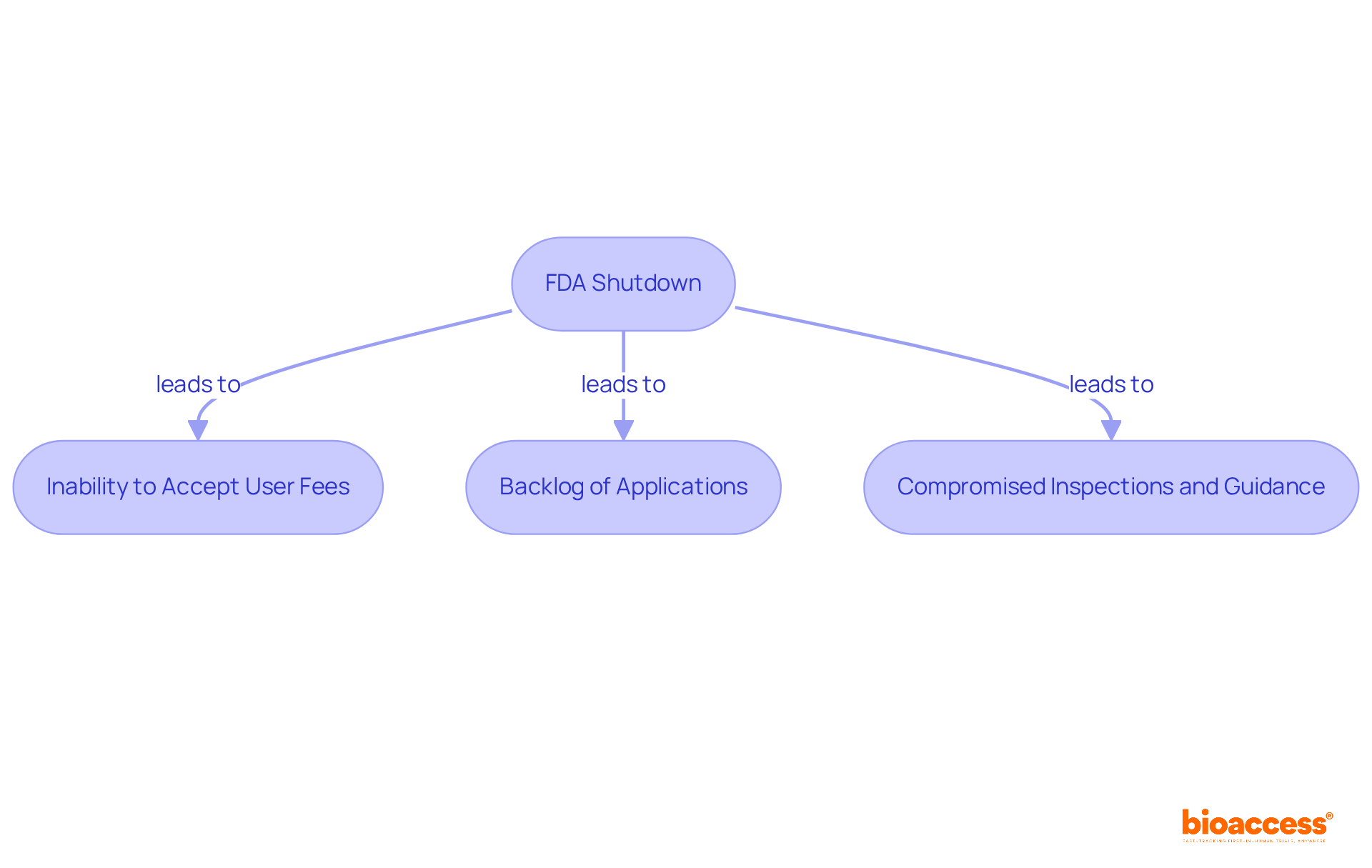
The challenges are exacerbated by the FDA shutdown, which prevents the acceptance of new testing submissions or fees, as evidenced in the current environment where reviews and approvals of medications are indefinitely stalled.
Participants in clinical research must understand these dynamics as they navigate the obstacles presented by government halts, which can have lasting effects on the advancement of medical technologies and patient care.
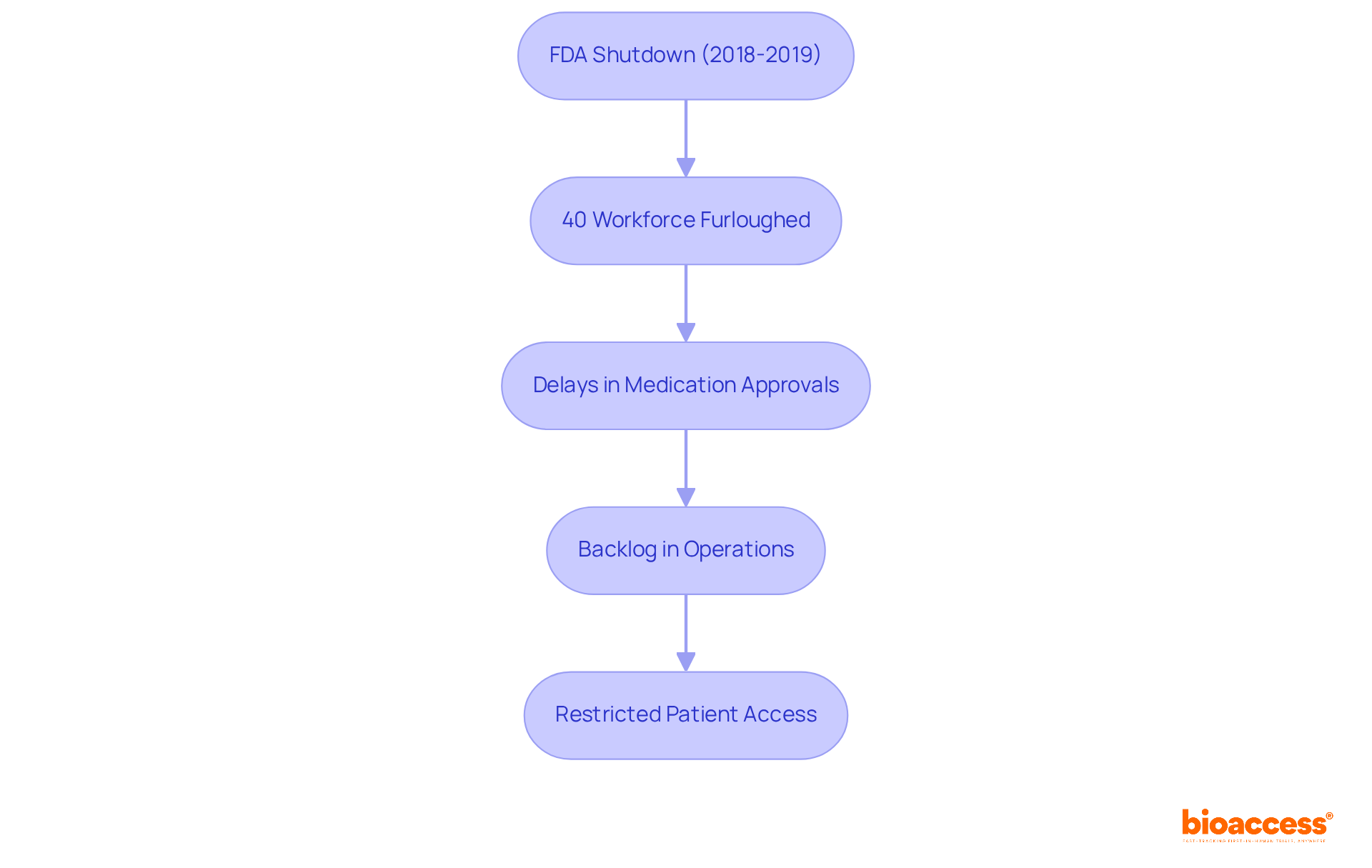
An FDA shutdown can lead to significant delays in the medication approval processes conducted by the government. For example, during the FDA shutdown in 2018-2019:
Stakeholders must recognize that these delays can have cascading effects on research trials, necessitating adjustments to their timelines and expectations based on the FDA's operational capacity during such interruptions.
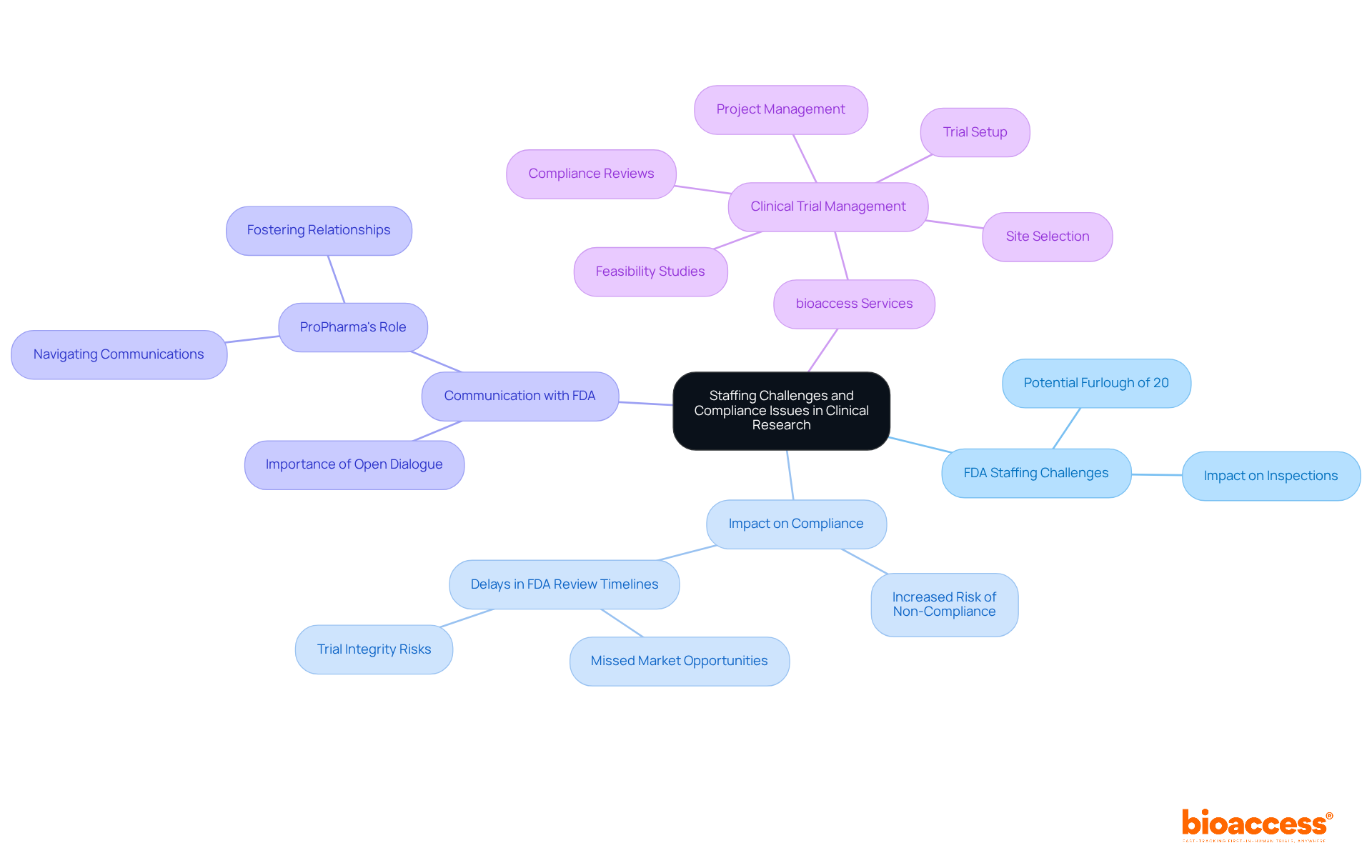
During government shutdowns, the FDA faces significant staffing challenges that can lead to compliance issues in research studies. With nearly 20% of FDA personnel potentially furloughed, timely inspections and evaluations become increasingly difficult, heightening the risk of non-compliance among trial sponsors. Delays in FDA review timelines can result in missed market opportunities, complicating the landscape for clinical research. For instance, if a trial site remains unexamined due to staffing shortages, it may fail to meet the necessary regulatory standards, jeopardizing the integrity of the study. Moreover, the uncertainty surrounding staffing levels can induce anxiety among researchers and sponsors as they navigate the complexities of compliance during these turbulent times.
To mitigate these risks, it is crucial for research teams to remain vigilant and adaptable, ensuring compliance even amid these challenges. Effective communication with the FDA is paramount; as ProPharma emphasizes, fostering a positive relationship and maintaining open dialogue with the Agency is essential for the success of pharmaceutical programs. Furthermore, familiarity with the FDA's new Manual of Policies and Procedures (MAPP) concerning drug-device combination products can offer valuable insights into compliance standards during shutdowns.
At bioaccess, our comprehensive clinical trial management services—including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting—are tailored to assist clinical research teams in effectively navigating these obstacles. Under the leadership of Katherine Ruiz, an expert in regulatory affairs for medical devices and in vitro diagnostics in Colombia, we ensure that our clients are well-prepared to tackle the intricacies of compliance, even during uncertain times.
The implications of FDA shutdowns extend far beyond immediate operational disruptions; they pose significant challenges to the clinical research landscape. Understanding the FDA's role and the consequences of its shutdowns is crucial for stakeholders in the pharmaceutical and medical device industries. These interruptions not only delay drug approvals but also create a ripple effect that can hinder innovation and patient access to vital therapies.
Key insights highlight the staggering impact of furloughs on FDA staffing, leading to backlogs in drug applications and heightened compliance risks for clinical trials. The inability to accept new user fees and conduct timely inspections can stall research progress and complicate compliance for trial sponsors. As the FDA's operational capacity diminishes during shutdowns, it becomes imperative for research teams to remain adaptable, maintain open communication with the agency, and stay informed about evolving compliance standards.
In light of these challenges, it is essential for industry stakeholders to proactively prepare for potential government interruptions. By fostering strong relationships with the FDA and leveraging resources such as clinical trial management services, researchers can navigate the complexities of compliance and ensure the integrity of their studies. Ultimately, acknowledging the significant impact of FDA shutdowns on clinical research operations empowers stakeholders to advocate for more stable regulatory environments, ensuring that patient care and medical advancements remain a priority.
What is the role of the FDA?
The Food and Drug Administration (FDA) oversees the safety and efficacy of food, medications, and medical devices in the United States.
What happens during an FDA shutdown?
During an FDA shutdown, operations can face significant interruptions, including furloughs of staff and delays in medication approvals and inspections.
What was the impact of the FDA shutdown from 2018 to 2019?
Nearly 40% of the FDA's workforce was furloughed, leading to substantial delays in medication approvals and inspections, creating a backlog that hindered the FDA's efficiency and impacted the pharmaceutical and medical device industries.
How does an FDA shutdown affect patient access to therapies?
An FDA shutdown restricts patient access to new therapies and innovations due to delays in the approval process.
What challenges do clinical research participants face during an FDA shutdown?
Clinical research participants must navigate obstacles such as stalled reviews and approvals of medications, which can have lasting effects on the advancement of medical technologies and patient care.
What specific operations are halted during an FDA shutdown?
During an FDA shutdown, the acceptance of new testing submissions or fees is prevented, causing indefinite delays in reviews and approvals of medications.