


The article examines the critical role of healthy volunteer clinical trials, underscoring their phases, benefits, and ethical considerations. Healthy volunteers are pivotal in early-phase research, as they establish safety benchmarks for new treatments. Furthermore, the article provides insights into the ethical protocols that protect participants and uphold the integrity of the studies. This exploration is essential for understanding the landscape of clinical research and the collaborative efforts required to address key challenges.
Understanding the intricacies of healthy volunteer clinical trials unveils a critical component of medical research that often goes unnoticed. These trials not only pave the way for groundbreaking therapies but also offer healthy participants a unique opportunity to contribute to the advancement of healthcare while potentially benefiting from enhanced medical oversight.
However, the ethical landscape surrounding these trials raises significant questions about participant safety and informed consent. What are the implications of their involvement, and how do researchers ensure that the benefits outweigh the risks?
Delving into the phases, benefits, and ethical considerations of these trials reveals a complex interplay of science, safety, and societal advancement.
Clinical studies represent meticulously designed research investigations that assess the safety and efficacy of new medical interventions, including drugs, devices, and treatment protocols. The involvement of healthy volunteer clinical trials is crucial, particularly during early-phase research, as they provide vital baseline information necessary for evaluating the impacts of these interventions. Their role is essential in establishing the safety profile of new treatments before testing on patients with specific conditions.
By serving as a control group in healthy volunteer clinical trials, healthy volunteers enable researchers to discern how a drug or treatment interacts with a normal physiological system, which is vital for developing effective therapies. Statistics indicate that:
The knowledge acquired from fit participants not only enhances the understanding of treatment impacts but also informs ethical considerations in research design, ensuring that the safety and welfare of individuals are prioritized.
At bioaccess®, we specialize in comprehensive research management services, including:
This ensures that healthy volunteer clinical trials are optimized for the best outcomes. Our expertise in navigating the complexities of clinical studies in Latin America allows us to expedite the process, delivering FDA-ready data that reduces both expenses and time. This innovative approach not only enhances the efficiency of patient recruitment but also ensures that the safety and efficacy of new medical interventions are thoroughly evaluated.
Clinical trials are systematically categorized into four primary phases, each serving a distinct purpose in the drug development process:
Phase I: This foundational phase is dedicated to evaluating the safety and tolerability of a new drug or treatment. Usually involving 20 to 100 fit participants, the emphasis is on comprehending how the body processes the medication and recognizing any possible side effects. The participation of well individuals in healthy volunteer clinical trials is essential in setting safety standards that guide later stages. With bioaccess®, treatment-naive cardiology or neurology cohorts can be enrolled 50% faster than traditional Western sites, significantly enhancing the efficiency of this phase.
Phase II: In this phase, the treatment is administered to a larger cohort of participants, usually ranging from 100 to 300. This stage aims to further assess the drug's efficacy and side effects. While healthy volunteer clinical trials may still include healthy volunteers, this phase increasingly incorporates patients who have the condition the drug intends to treat. Successful Phase II studies often depend on insights acquired from earlier stages, ensuring a thorough assessment of the treatment's potential. The estimated completion rate for Phase II clinical studies was 81.1% in 2018, highlighting the importance of thorough evaluation during this stage.
Phase III: This phase is characterized by extensive participation, often involving thousands of individuals. The primary objectives are to confirm the treatment's effectiveness, monitor side effects, and compare it against existing therapies. At this stage, healthy volunteer clinical trials are less likely to include healthy volunteers, as the focus shifts to patients with the targeted condition. bioaccess® facilitates this process by providing FDA-ready data, ensuring no rework or delays, which can lead to $25K savings per patient. The completion rate for Phase III studies was estimated at 84.9% in 2018, indicating the rigorous standards applied during this phase.
Phase IV: Following FDA approval, Phase IV studies are conducted to monitor long-term effects and gather additional data on the drug's risks, benefits, and optimal usage. Healthy volunteer clinical trials may include participants in specific studies aimed at assessing broader impacts, thereby contributing valuable information to the ongoing evaluation of the treatment. As of May 2023, 53% of all registered studies are carried out beyond the US, highlighting the worldwide landscape of research and the varied involvement of healthy participants.
Healthy volunteer clinical trials play a crucial part in the initial stages of research studies, especially in Phase I, where their involvement is vital for setting safety benchmarks that inform the advancement of new treatments. The existing method of assessing the safety of new medications significantly relies on social disparities to aid in recruitment and enrollment for these studies. As the scenery of medical studies progresses, there is a rising complexity and a heightened focus on the ethical aspects regarding participant involvement.

Participating in clinical trials as a healthy volunteer presents several significant benefits that are both impactful and rewarding:
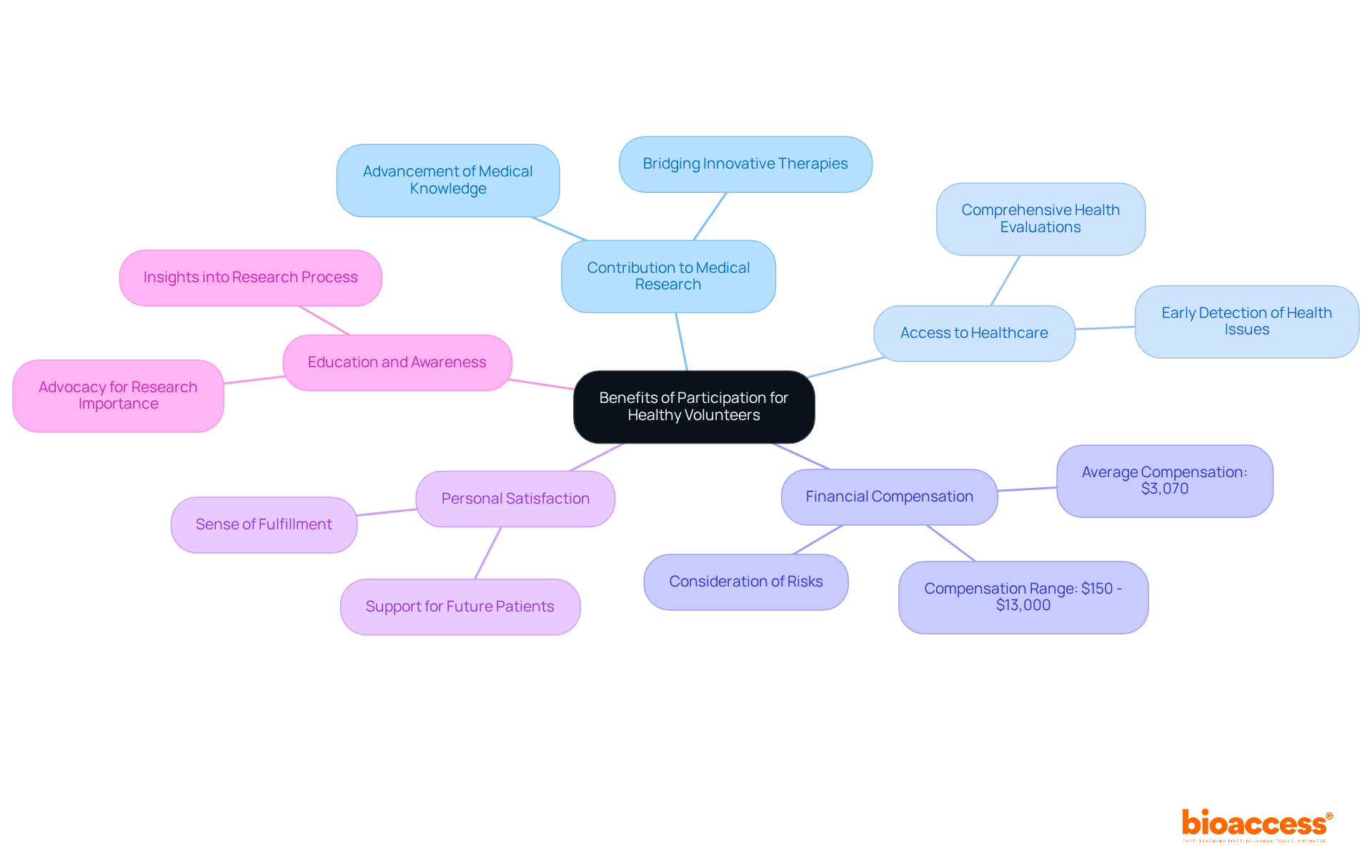
Contribution to Medical Research: Volunteers are pivotal in advancing medical knowledge and developing new treatments that can profoundly affect society. Their involvement is crucial in bridging the gap between innovative therapies and their practical applications in healthcare.
Access to Healthcare: Participants often undergo comprehensive health evaluations and ongoing monitoring during the study. This can facilitate the early detection of potential health issues, offering an added layer of healthcare that may not be accessible otherwise.
Financial Compensation: A considerable number of healthy participants receive financial compensation for their time and travel. Research indicates that the average compensation for trial participation is approximately $3,070, with many trials providing payments that address the logistical burden and associated risks.
Personal Satisfaction: Numerous individuals report a sense of fulfillment from contributing to scientific progress. Their participation not only aids in the development of new therapies but also supports the broader objective of enhancing healthcare outcomes for future patients.
Education and Awareness: Volunteers acquire valuable insights into the research process, which enhances their understanding of medical advancements. This knowledge fosters greater awareness of the significance of research in healthcare, enabling participants to advocate for studies and their benefits.
In summary, healthy volunteer clinical trials play an essential role in medical research, and their participation can lead to improved health outcomes, both for themselves and the wider community.
Ethical considerations and safety protocols are fundamental in healthy volunteer clinical trials, as they safeguard participants and ensure the integrity of the research. Key aspects include:
Informed Consent: Participants must be fully informed about the study's purpose, procedures, risks, and benefits before agreeing to take part. This process ensures that volunteers make educated decisions, with studies indicating that 74.7% of participants understood the nature of the study and 75.8% recognized their right to withdraw at any time.
Risk-Benefit Evaluation: Researchers must assess the potential risks in comparison to the anticipated advantages of the study. Only healthy volunteer clinical trials with a favorable risk-benefit ratio are approved, ensuring that ethical standards are upheld.
Monitoring and Oversight: Institutional Review Boards (IRBs) supervise clinical studies to ensure ethical standards are upheld. They assess study protocols and oversee ongoing experiments for adherence to ethical standards. Furthermore, bioaccess offers extensive project management and monitoring services to guarantee compliance with these standards during the study, including feasibility assessments and site selection.
Participant safety is crucial during healthy volunteer clinical trials, requiring ongoing observation to quickly detect any adverse effects. Safety protocols are in place to minimize risks and ensure participant well-being. The expertise of bioaccess in trial setup and management, including obtaining necessary import permits, further enhances participant safety by ensuring that all necessary precautions are taken.
Confidentiality: Researchers are obligated to protect the privacy of participants and handle their data responsibly, ensuring that personal information is kept confidential throughout the study. Compliance reviews conducted by bioaccess help ensure that all data handling practices meet regulatory requirements, reinforcing the commitment to participant confidentiality.

Healthy volunteer clinical trials are a cornerstone of medical research, providing essential data that informs the development of new treatments. Their involvement establishes safety benchmarks and enhances the understanding of how interventions interact with the human body. This foundational role underscores the importance of healthy volunteers in the early phases of clinical studies, where their contributions are invaluable for ensuring the efficacy and safety of future therapies.
The structured phases of clinical trials highlight how healthy volunteers primarily participate in Phase I studies to assess safety while also contributing insights that benefit subsequent phases. The benefits of participation extend beyond research contributions, offering volunteers access to healthcare, financial compensation, and personal fulfillment from aiding scientific progress. Ethical considerations and safety protocols are paramount, ensuring that volunteers are well-informed and protected throughout their involvement.
Ultimately, the significance of healthy volunteers in clinical trials cannot be overstated. Their participation is vital for advancing medical knowledge and fostering a healthcare environment that prioritizes safety and ethical standards. Engaging in clinical trials represents an opportunity for individuals to make a meaningful impact on future medical advancements while also gaining personal health benefits. Embracing this role can lead to a healthier society, with innovative treatments developed through the dedication and courage of those who volunteer.
What are clinical trials?
Clinical trials are meticulously designed research investigations that assess the safety and efficacy of new medical interventions, including drugs, devices, and treatment protocols.
Why are healthy volunteers important in clinical trials?
Healthy volunteers are crucial in early-phase research as they provide vital baseline information necessary for evaluating the impacts of new interventions and help establish the safety profile of treatments before they are tested on patients with specific conditions.
How do healthy volunteers contribute to clinical research?
By serving as a control group, healthy volunteers enable researchers to understand how a drug or treatment interacts with a normal physiological system, which is essential for developing effective therapies.
What do statistics say about participants' experiences in clinical trials?
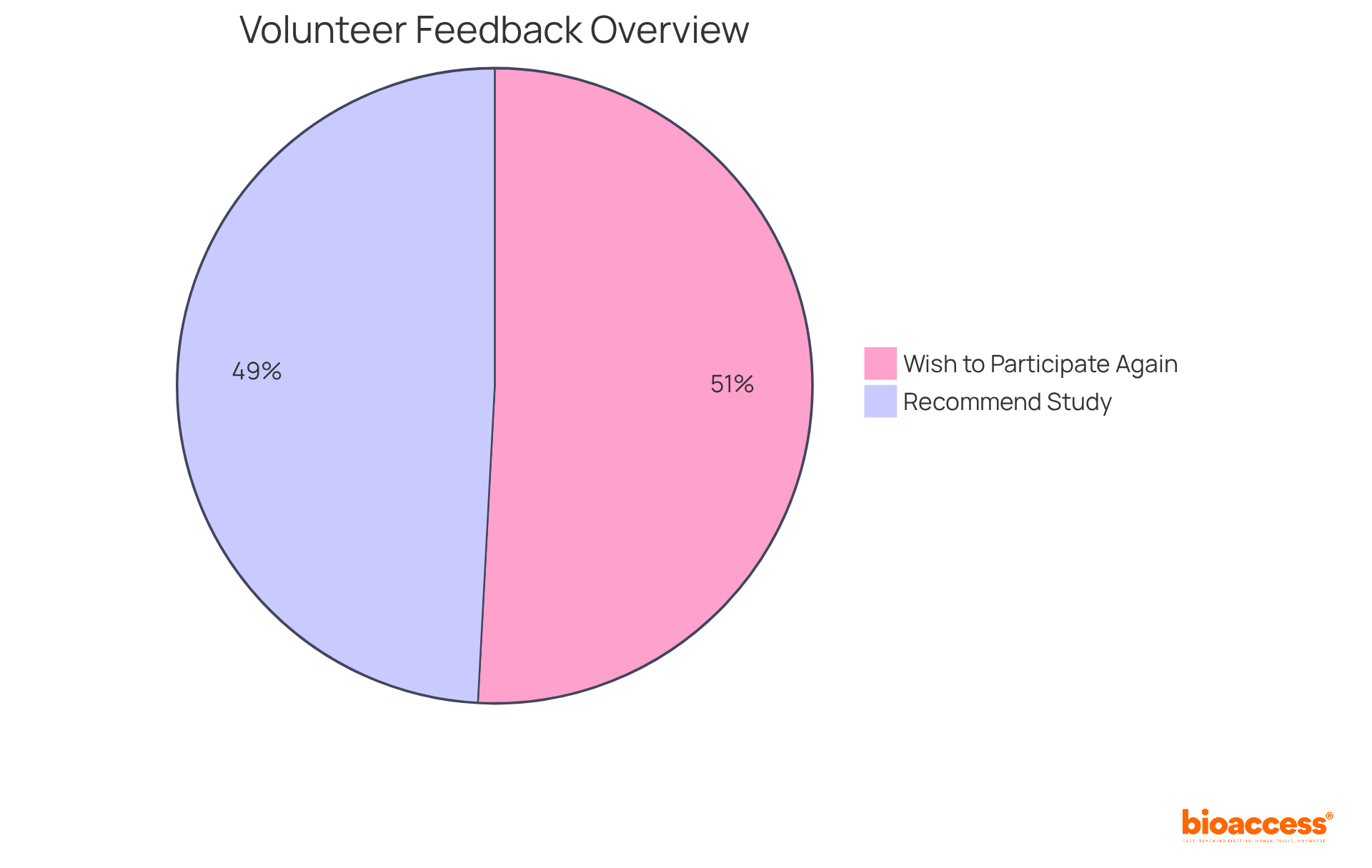
Statistics indicate that 87% of participants would recommend joining the study to friends or family, and 90% expressed a desire to engage in experiments again, highlighting the favorable experiences associated with their involvement.
What services does bioaccess® provide in relation to clinical trials?
Bioaccess® specializes in comprehensive research management services, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF).
How does bioaccess® enhance the clinical trial process?
Bioaccess® navigates the complexities of clinical studies in Latin America to expedite the process, delivering FDA-ready data that reduces both expenses and time while ensuring thorough evaluation of the safety and efficacy of new medical interventions.