


This article elucidates the significance of Interactive Response Technology (IRT) in clinical trials, emphasizing its mechanisms, benefits, and seamless integration within clinical study processes. IRT is pivotal as it automates critical functions such as subject randomization and data management. This automation not only enhances operational efficiency but also minimizes errors and ensures adherence to regulatory standards. Collectively, these advantages contribute to the acceleration and reliability of clinical trials, underscoring the necessity for clinical researchers to embrace IRT in their methodologies.
The landscape of clinical trials is undergoing a transformative shift, with Interactive Response Technology (IRT) emerging as a cornerstone for enhancing operational efficiency and compliance. As the demand for faster, more reliable clinical research grows, it is essential for stakeholders to understand the mechanisms and benefits of IRT in order to streamline their processes. However, with rapid advancements in technology, researchers must consider how to effectively integrate IRT systems to overcome existing challenges and ensure the integrity of their studies.
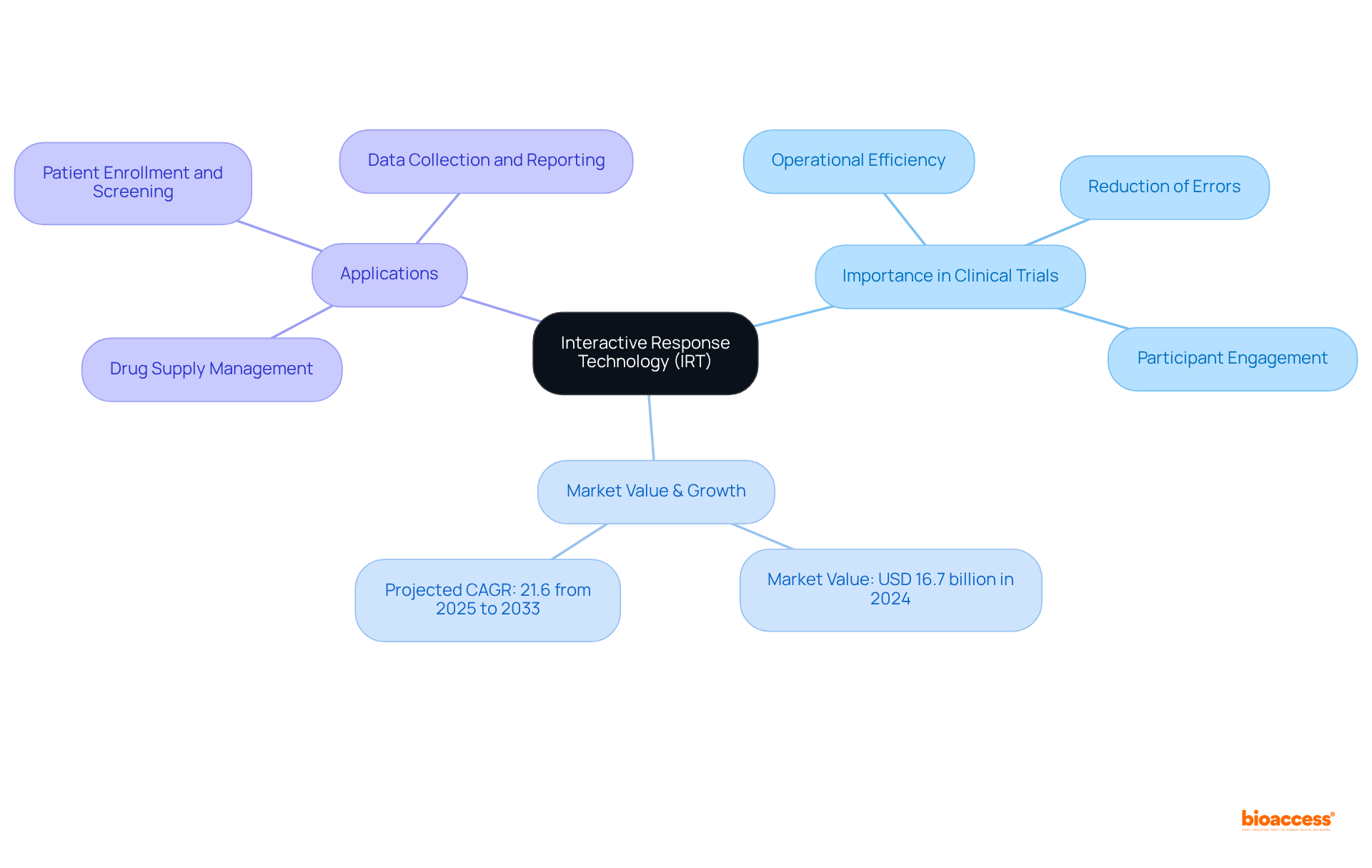
The IRT clinical trial represents a pivotal advancement in clinical study management, automating essential processes such as subject randomization, drug supply oversight, and data collection. The significance of the IRT clinical trial in clinical research cannot be overstated, as it enhances operational efficiency, reduces the likelihood of manual errors, and ensures compliance with regulatory standards. By streamlining these processes, the IRT clinical trial empowers clinical researchers to concentrate on critical tasks, thereby improving outcomes for participants and accelerating study timelines.
The IRT market was valued at USD 16.7 billion in 2024, with projections indicating a compound annual growth rate (CAGR) of 21.6% from 2025 to 2033. This remarkable growth underscores the increasing reliance on IRT systems to facilitate seamless communication among stakeholders and to ensure the effective and efficient operation of studies. Practical applications of IRT have demonstrated its effectiveness in enhancing study efficiency; for instance, organizations such as bioaccess® have achieved ethical approvals in a mere 4-6 weeks and accelerated participant enrollment by 50% compared to traditional methods.
Such developments highlight IRT clinical trial systems' indispensable role as the backbone of modern clinical studies, particularly in enhancing participant engagement and reducing study costs. As the landscape of clinical research evolves, the collaboration between innovative technologies like IRT and dedicated researchers will be crucial in overcoming existing challenges and driving the future of IRT clinical trials.
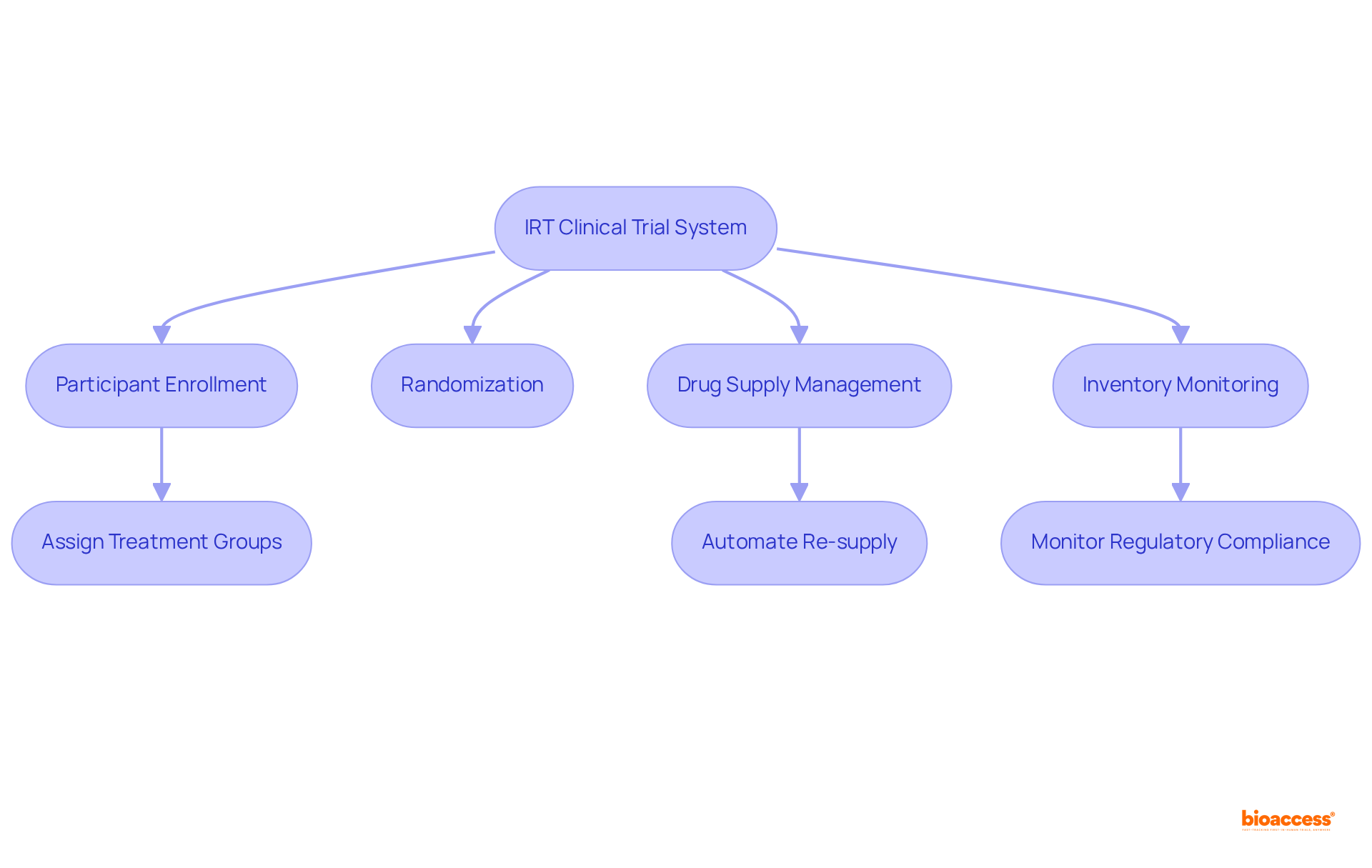
IRT clinical trial systems operate through a sophisticated blend of web-based and mobile interfaces, enabling real-time data management crucial for clinical trials. This operational framework encompasses essential modules for participant enrollment, randomization, and drug supply management. Upon patient enrollment, the IRT system assigns individuals to treatment groups based on predefined criteria, ensuring unbiased randomization and adherence to study protocols. Furthermore, IRT consistently oversees inventory levels of investigational products, automating re-supply triggers to prevent shortages and uphold study integrity.
This seamless integration of functionalities streamlines information collection and management while enhancing integrity by minimizing human intervention, significantly reducing the potential for errors. IRT enhances adherence to regulatory standards such as Good Clinical Practice (GCP) and 21 CFR Part 11, ensuring secure and accurate study data. Statistics suggest that IRT clinical trial studies, particularly those supported by bioaccess®, reach patient enrollment 50% quicker than conventional methods, underscoring the technology's crucial role in expediting clinical research processes.
Additionally, clients can save upwards of $20,000 a month through automated supply optimization, highlighting the financial benefits of implementing IRT. Looking forward, advancements in IRT technology, including AI-driven supply forecasting and predictive analytics, are anticipated to further improve efficiency in studies.
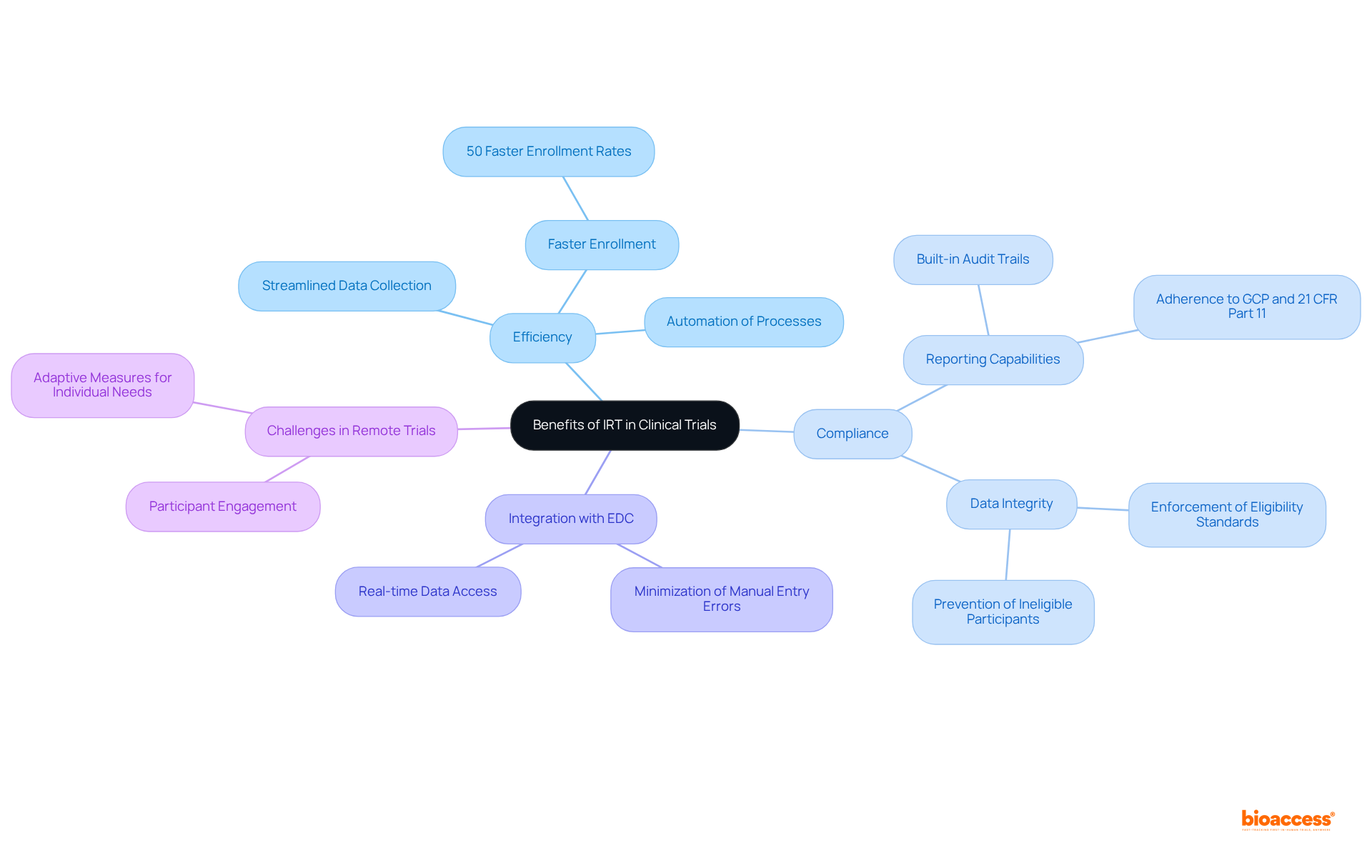
The incorporation of Interactive Response Technology (IRT) clinical trial in clinical studies presents substantial benefits, particularly in enhancing efficiency and ensuring adherence. By automating essential processes such as participant randomization and medication supply management, IRT significantly reduces the time and resources required for study execution. This automation leads to quicker participant enrollment and accelerated information gathering, effectively shortening study timelines. Notably, bioaccess® facilitates enrollment rates that are 50% faster than traditional markets, underscoring its impact on operational efficiency.
Moreover, IRT systems come equipped with comprehensive reporting capabilities that uphold compliance with regulatory standards, including Good Clinical Practice (GCP) and 21 CFR Part 11. The built-in audit trails of IRT enhance transparency and accountability, essential for fostering trust among stakeholders. For instance, case studies like 'Site and Patient Management with IRT' illustrate that IRT not only simplifies patient enrollment and visit timelines but also enforces eligibility standards, thereby enhancing information integrity and compliance.
Furthermore, the integration of IRT with Electronic Data Capture (EDC) systems further refines clinical study management by streamlining data collection and minimizing errors. As clinical studies evolve in complexity, the role of IRT clinical trial in ensuring regulatory compliance and operational efficiency becomes increasingly vital. It is also crucial to acknowledge the challenges in remote studies, such as participant involvement, which IRT can help mitigate through its adaptive measures tailored to individual participants’ needs.
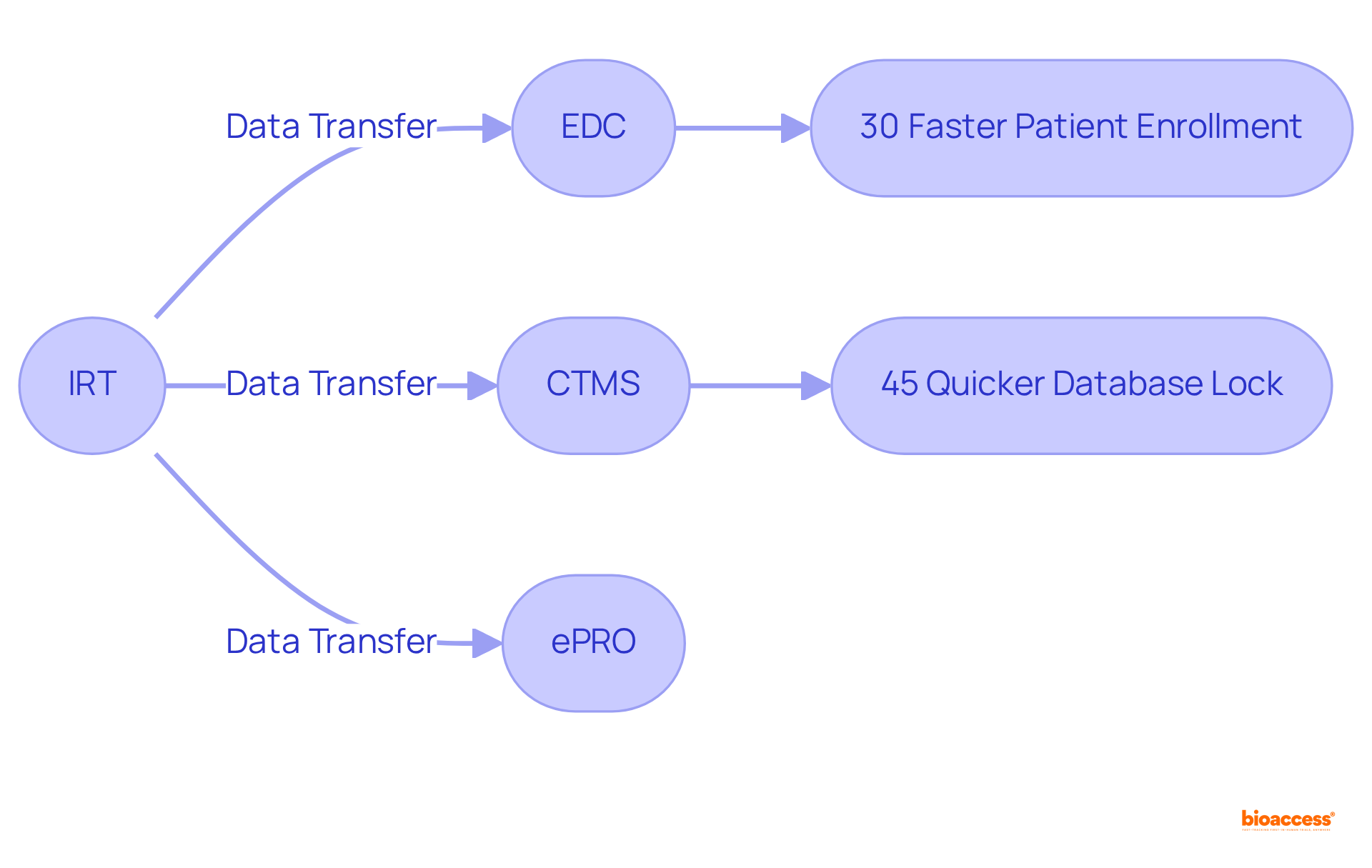
IRT clinical trial systems play a crucial role in the seamless integration with other clinical trial technologies, including Electronic Data Capture (EDC), Clinical Trial Management Systems (CTMS), and electronic patient-reported outcomes (ePRO). This integration facilitates the transfer of information among systems, ensuring that all stakeholders have access to real-time details.
For example, when an individual is randomized through the IRT, the information is automatically refreshed in the EDC, significantly reducing the need for manual entry and minimizing errors. Statistics reveal that clinical studies employing integrated, automated management workflows complete patient enrollment 30% faster and achieve database lock in 45% less time compared to studies that utilize fragmented systems. A prominent pharmaceutical company reported a 40% reduction in entry errors during a Phase III oncology study by leveraging an integrated management platform.
Additionally, IRT enhances supply chain management by providing valuable insights into inventory levels and usage patterns, enabling proactive adjustments. By streamlining these processes, the IRT clinical trial not only boosts operational efficiency but also enhances the overall quality of data collected during clinical studies. It acts as a centralized platform for stakeholders to monitor and control trial operations, underscoring the necessity of collaboration among all parties involved for successful integration.
Interactive Response Technology (IRT) has emerged as a transformative force in the realm of clinical trials, streamlining essential processes and enhancing the overall efficiency of research studies. By automating participant randomization, drug supply management, and data collection, IRT systems enable researchers to concentrate on critical aspects of their trials, ultimately leading to enhanced outcomes for participants and a quicker pace of research. The significance of IRT in clinical trial management is paramount, as it serves as a vital tool in navigating the complexities of modern clinical research.
This article underscores several key advantages of IRT, including its capacity to:
Statistics reveal that IRT can accelerate patient enrollment by up to 50% and significantly decrease operational costs, making it evident that the integration of IRT systems is not merely beneficial but essential for successful clinical trials. Furthermore, the seamless collaboration between IRT and other clinical technologies, such as Electronic Data Capture and Clinical Trial Management Systems, highlights the necessity of a cohesive approach to data management and operational efficiency.
As the landscape of clinical research continues to evolve, embracing technologies like IRT will be crucial for overcoming challenges and driving innovation. Stakeholders in the clinical trial ecosystem are urged to recognize the transformative potential of IRT systems and prioritize their integration strategically. By doing so, researchers can not only enhance the quality and integrity of their studies but also contribute significantly to the advancement of medical science as a whole.
What is Interactive Response Technology (IRT) in the context of clinical trials?
Interactive Response Technology (IRT) is a pivotal advancement in clinical study management that automates essential processes such as subject randomization, drug supply oversight, and data collection.
Why is IRT important in clinical trials?
IRT is important because it enhances operational efficiency, reduces the likelihood of manual errors, ensures compliance with regulatory standards, and allows clinical researchers to focus on critical tasks, thereby improving outcomes for participants and accelerating study timelines.
What was the market valuation of IRT in 2024 and what are its projected growth rates?
The IRT market was valued at USD 16.7 billion in 2024, with a projected compound annual growth rate (CAGR) of 21.6% from 2025 to 2033.
How does IRT improve communication among stakeholders in clinical trials?
IRT facilitates seamless communication among stakeholders, ensuring the effective and efficient operation of studies, which is crucial for successful clinical trial management.
Can you provide an example of IRT's effectiveness in clinical trials?
An example of IRT's effectiveness is that organizations like bioaccess® have achieved ethical approvals in just 4-6 weeks and accelerated participant enrollment by 50% compared to traditional methods.
What role does IRT play in enhancing participant engagement and reducing study costs?
IRT systems serve as the backbone of modern clinical studies, enhancing participant engagement and helping to reduce study costs through streamlined processes and improved efficiency.
How is the collaboration between IRT and researchers important for the future of clinical trials?
The collaboration between innovative technologies like IRT and dedicated researchers is crucial for overcoming existing challenges in clinical research and driving the future of IRT clinical trials.