


MAPS Medical Affairs functions as a pivotal link between clinical development and commercial operations within the life sciences sector, emphasizing effective communication and stakeholder engagement to improve health outcomes. This article highlights that its essential functions—medical communication, clinical trial support, and real-world evidence generation—are crucial for navigating the complexities of modern healthcare. These roles not only facilitate the successful implementation of innovative treatments but also address key challenges faced in clinical research.
Collaboration among stakeholders is vital, reinforcing the need for a cohesive approach to enhance health outcomes.
The landscape of healthcare is rapidly evolving, positioning MAPS Medical Affairs as a cornerstone in bridging clinical development and commercial success. This function not only enhances communication about medical products but also plays a critical role in equipping healthcare professionals with the necessary information to improve patient outcomes.
As the industry approaches 2025, pressing questions emerge regarding how MAPS Medical Affairs will adapt to the increasing demands for real-world evidence and patient-centric strategies. What challenges and opportunities lie ahead for this vital component of the healthcare ecosystem?
The role of maps medical affairs is pivotal in the life sciences sector, acting as a crucial link between clinical development and commercial operations. This function maps medical affairs by encompassing a range of activities that ensure medical products are effectively communicated, understood, and utilized in clinical practice. The significance of MAPS Health Services is underscored by its commitment to enhancing health outcomes through the delivery of precise information that maps medical affairs for medical practitioners, thereby supporting the effective launch and acceptance of groundbreaking treatments. By fostering collaboration among diverse stakeholders, MAPS Healthcare maps medical affairs to advance health knowledge and improve healthcare delivery.
Looking ahead to 2025, the anticipated impact of MAPS Healthcare Operations on individual outcomes is substantial, particularly as it increasingly harnesses Real-World Evidence (RWE) to inform clinical trial designs and reimbursement strategies. This evolution facilitates a deeper understanding of treatment effectiveness in real-world settings, ultimately leading to improved access to innovative therapies for patients. For instance, effective strategies employed by teams that maps medical affairs include engaging professionals early in the clinical trial process and utilizing insights to tailor communication approaches. Such efforts not only enhance disease awareness but also map medical affairs to ensure that the information disseminated is relevant and advantageous to both medical professionals and patients.
Expert insights affirm that the evolving role of maps medical affairs is critical for navigating the complexities of modern health services. By emphasizing patient-centric approaches and integrating compliance considerations, maps medical affairs can adeptly address the needs of both patients and providers. This strategic focus is essential for maximizing the impact of medical affairs initiatives and maps medical affairs to ensure alignment with broader health objectives.
In the realm of advancing medical device trials, bioaccess® exemplifies this function by offering a structured methodology that encompasses trial set-up, start-up, and approval processes, along with thorough reporting on study status and adverse events. This approach effectively tackles the challenges encountered by medical device startups, including regulatory hurdles and recruitment difficulties, while also expediting site activation and ensuring compliance across diverse regions such as LATAM, the Balkans, and Australia. By leveraging over 20 years of expertise in Medtech clinical trials, bioaccess® significantly supports Medtech and biopharma startups, ultimately facilitating successful acquisitions through ethical clinical data and strategic planning.

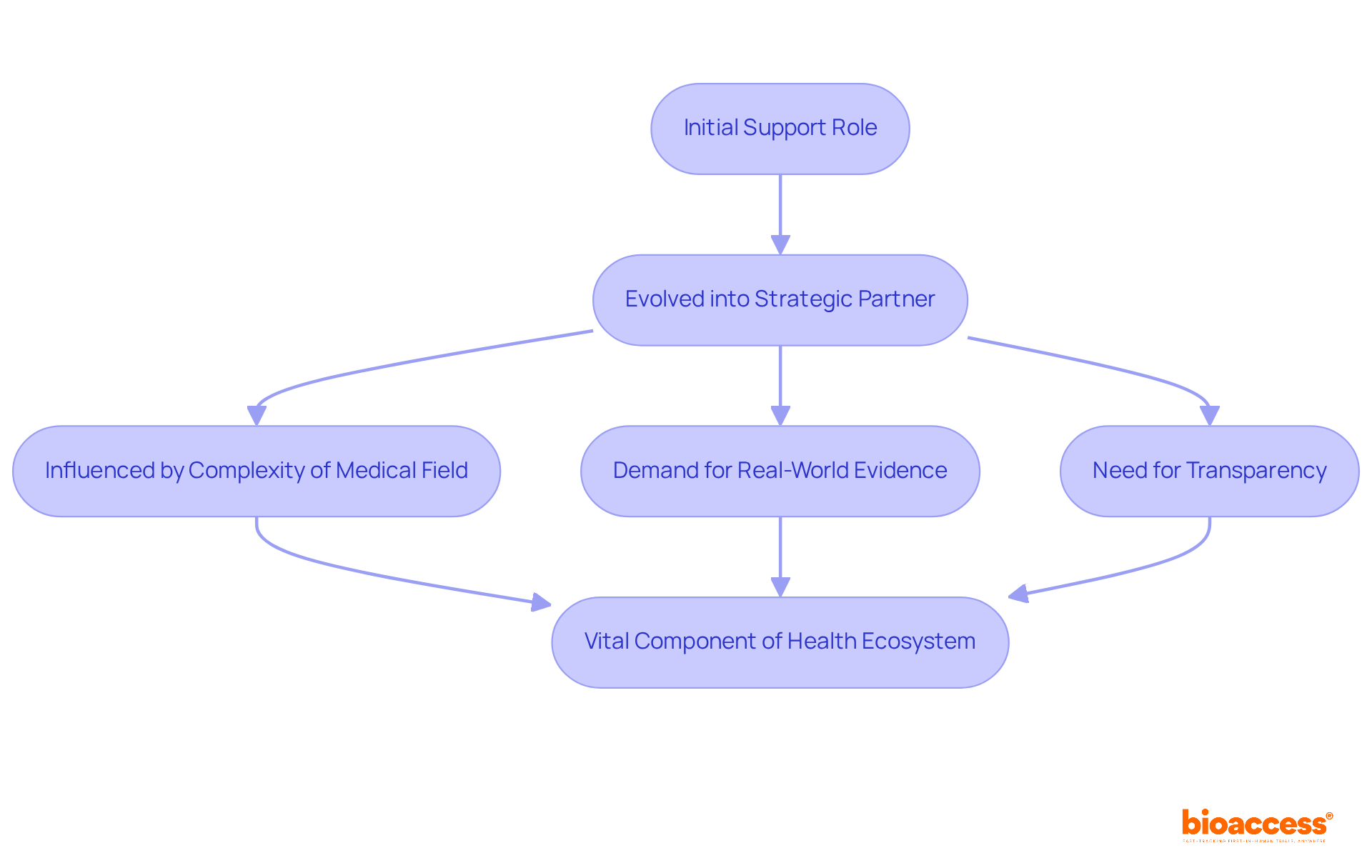
The development of MAPS Services originates from a support role that primarily provided scientific information to medical professionals. Over time, it has evolved into a strategic partner in the drug development process, significantly shaping clinical development plans and commercialization strategies. This transformation has been propelled by the increasing complexity of the medical field, the necessity for real-world evidence, and a rising demand for transparency and accountability in the pharmaceutical sector.
Today, the role of MAPS medical affairs is recognized as a vital component of the health ecosystem, actively influencing decision-making processes at various levels. The focus on integrating insights from diverse stakeholders, including patients and payers, further underscores its essential role in enhancing health outcomes.
Key functions of MAPS Medical Affairs encompass several vital areas:
Medical Communication: Delivering accurate and timely information to healthcare professionals and stakeholders is essential for informed decision-making. Effective communication strategies are crucial, as evidenced by the emphasis on understanding audience motivations and needs in scientific storytelling.
Clinical Trial Support: Interacting with researchers and locations is essential for promoting participant recruitment and retention in clinical studies. Recent initiatives have showcased the importance of flexibility in storytelling approaches to enhance engagement and educational impact during trials.
Real-World Evidence Generation: Collecting and analyzing data from real-world settings supports product value propositions, allowing for a deeper understanding of treatment effectiveness and outcomes for individuals. This aligns with the growing need for meaningful impact metrics in Medical Affairs.
Stakeholder Engagement: Establishing connections with medical providers, payers, and advocacy groups for individuals ensures alignment on medical needs and product advantages. This engagement is critical for optimizing therapy access and value across various stakeholders.
Training and Education: Creating educational programs for medical professionals enhances their understanding of new therapies and treatment protocols. This function is increasingly acknowledged as essential for enhancing healthcare and outcomes.
Collectively, these functions enhance the effectiveness of Healthcare Operations in driving patient-centric outcomes and maps medical affairs to the evolving landscape of health services, highlighting the need for innovative approaches in medical communication and clinical trial support.

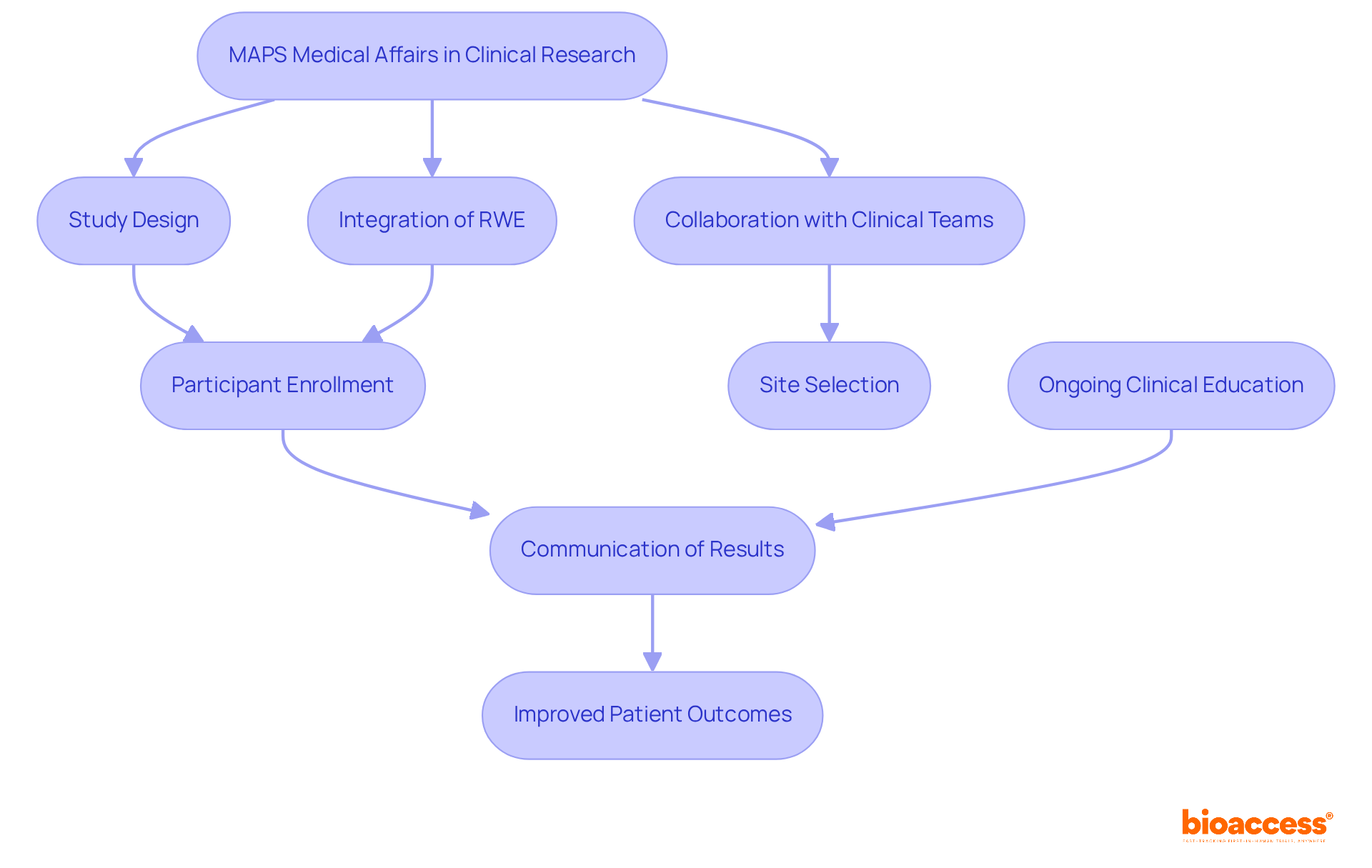
MAPS plays a pivotal role in clinical research, ensuring that studies are meticulously designed and executed with a focus on individual needs and real-world relevance. This commitment involves collaboration with clinical development teams to provide insights into diverse population groups, treatment pathways, and potential barriers to access. Furthermore, the integration of Real-World Evidence (RWE) into clinical trial design empowers MAPS Medical Affairs to effectively map medical affairs by reflecting authentic experiences in trials, thereby enhancing recruitment outcomes.
With bioaccess®'s advanced capabilities, clinical trials can achieve participant enrollment 50% faster, resulting in significant savings of $25K per individual through FDA-ready data that effectively eliminates rework and delays. These experts are instrumental in refining site selection and managing relationships with principal investigators (PIs), which significantly boosts participant engagement and recruitment efforts.
Additionally, MAPS professionals excel in communicating study results to stakeholders, ensuring that findings are transformed into actionable insights for health service providers. By bridging the gap between clinical research and practical application, the approach that maps medical affairs elevates the overall quality and relevance of clinical studies, ultimately leading to improved patient outcomes.
Ongoing Clinical Education (CME) programs further equip health sector professionals with the latest product information, enhancing the communication aspect of MAPS Clinical Functions' role. Through comprehensive clinical trial management services—including feasibility studies, compliance reviews, trial setup, and project management—MAPS Medical Affairs significantly contributes to economic growth and healthcare improvement within local communities.
The exploration of MAPS Medical Affairs reveals its essential role in bridging the gap between clinical development and commercial operations within the life sciences sector. By facilitating effective communication and collaboration among stakeholders, MAPS Medical Affairs significantly contributes to improving health outcomes and ensuring that innovative treatments reach patients efficiently.
The article underscores the evolution of MAPS Medical Affairs from a support role to a strategic partner in drug development. Key functions such as:
are vital in enhancing the effectiveness of healthcare operations. Each function plays a crucial part in addressing the complexities of modern healthcare, emphasizing the importance of patient-centric approaches and compliance considerations.
Reflecting on the insights provided, it is clear that the future of MAPS Medical Affairs will be shaped by its ability to adapt to the changing landscape of healthcare. As the integration of real-world evidence becomes increasingly prominent, stakeholders are encouraged to recognize the significance of MAPS Medical Affairs in optimizing patient care and clinical research outcomes. Embracing innovative strategies and fostering collaboration will not only enhance the impact of medical affairs initiatives but also pave the way for improved healthcare delivery in the years to come.
What is MAPS Medical Affairs and why is it important?
MAPS Medical Affairs serves as a crucial link between clinical development and commercial operations in the life sciences sector. It encompasses activities that ensure medical products are effectively communicated and utilized in clinical practice, ultimately enhancing health outcomes through the delivery of precise information to medical practitioners.
How does MAPS Healthcare impact health outcomes?
MAPS Healthcare is committed to improving health outcomes by fostering collaboration among diverse stakeholders and advancing health knowledge. This includes the effective launch and acceptance of groundbreaking treatments through tailored communication and disease awareness initiatives.
What role does Real-World Evidence (RWE) play in MAPS Healthcare Operations?
Real-World Evidence (RWE) is increasingly utilized by MAPS Healthcare Operations to inform clinical trial designs and reimbursement strategies. This approach enhances the understanding of treatment effectiveness in real-world settings, improving patient access to innovative therapies.
What strategies do teams in MAPS Medical Affairs employ?
Teams in MAPS Medical Affairs engage professionals early in the clinical trial process and use insights to tailor communication approaches. These strategies enhance disease awareness and ensure that the information shared is relevant and beneficial to both medical professionals and patients.
How does MAPS Medical Affairs address the needs of patients and providers?
MAPS Medical Affairs emphasizes patient-centric approaches and integrates compliance considerations to effectively address the needs of both patients and healthcare providers. This strategic focus maximizes the impact of medical affairs initiatives and aligns with broader health objectives.
What is bioaccess® and how does it relate to MAPS Medical Affairs?
Bioaccess® exemplifies the role of MAPS Medical Affairs by providing a structured methodology for advancing medical device trials. It includes trial set-up, start-up, approval processes, and thorough reporting, helping medical device startups navigate regulatory hurdles and recruitment challenges while ensuring compliance across various regions.