


This article delves into the critical assessment of market entry feasibility costs for Medtech innovators in Chile, underscoring the necessity of thoroughly evaluating:
It highlights the advantageous conditions present in Chile, including:
These factors collectively foster an environment that is conducive to successful market entry, making Chile an attractive destination for Medtech innovation.
Evaluating the market entry feasibility for Medtech innovators in Chile reveals a landscape abundant with potential yet laden with complexities.
How can companies effectively navigate the intricate web of regulations and competition to capitalize on this promising market?
Evaluating the market entry feasibility cost in Chile involves assessing the viability of a new sector, considering regulatory requirements, demand, competition, and operational costs. This evaluation holds particular significance in the nation, given its robust healthcare system and favorable regulatory framework for medical devices. By 2025, the healthcare sector is projected to reach a volume of approximately US$2.49 billion, propelled by rising consumer demand for advanced medical technologies and a stable economic environment.
Chile's healthcare system, characterized by a blend of public and private providers, facilitates access for Medtech innovators. The government has implemented policies to promote the use of advanced medical devices, including subsidies and support for research and development, thereby fostering a conducive environment for industry entry. Furthermore, relatively low import tariffs and the requirement for certification under international standards, such as EU CE marking and U.S. FDA approval, enhance Chile's attractiveness.
The potential for patient recruitment is further strengthened by Chile's well-educated workforce and a robust research and development ecosystem, which comprises universities and research institutes actively engaged in medical technology. This infrastructure not only supports the introduction of innovative products but also aligns with the growing trend towards digital health solutions, like telemedicine and wearable devices, which are increasingly in demand among consumers.
Expert opinions underscore that effective entry strategies should include forming partnerships with local distributors possessing strong networks in both public and private sectors. Engaging with these distributors can significantly enhance industry presence and ensure efficient after-sale service, which is highly valued in the Chilean healthcare landscape. Additionally, leveraging comprehensive clinical trial management services, such as those offered by bioaccess®, including Pilot Studies and Post-Market Clinical Follow-Up Studies, can substantially increase the likelihood of successful industry entry. Ultimately, understanding these elements is crucial for medical technology firms aiming to assess the market entry feasibility cost in Chile.
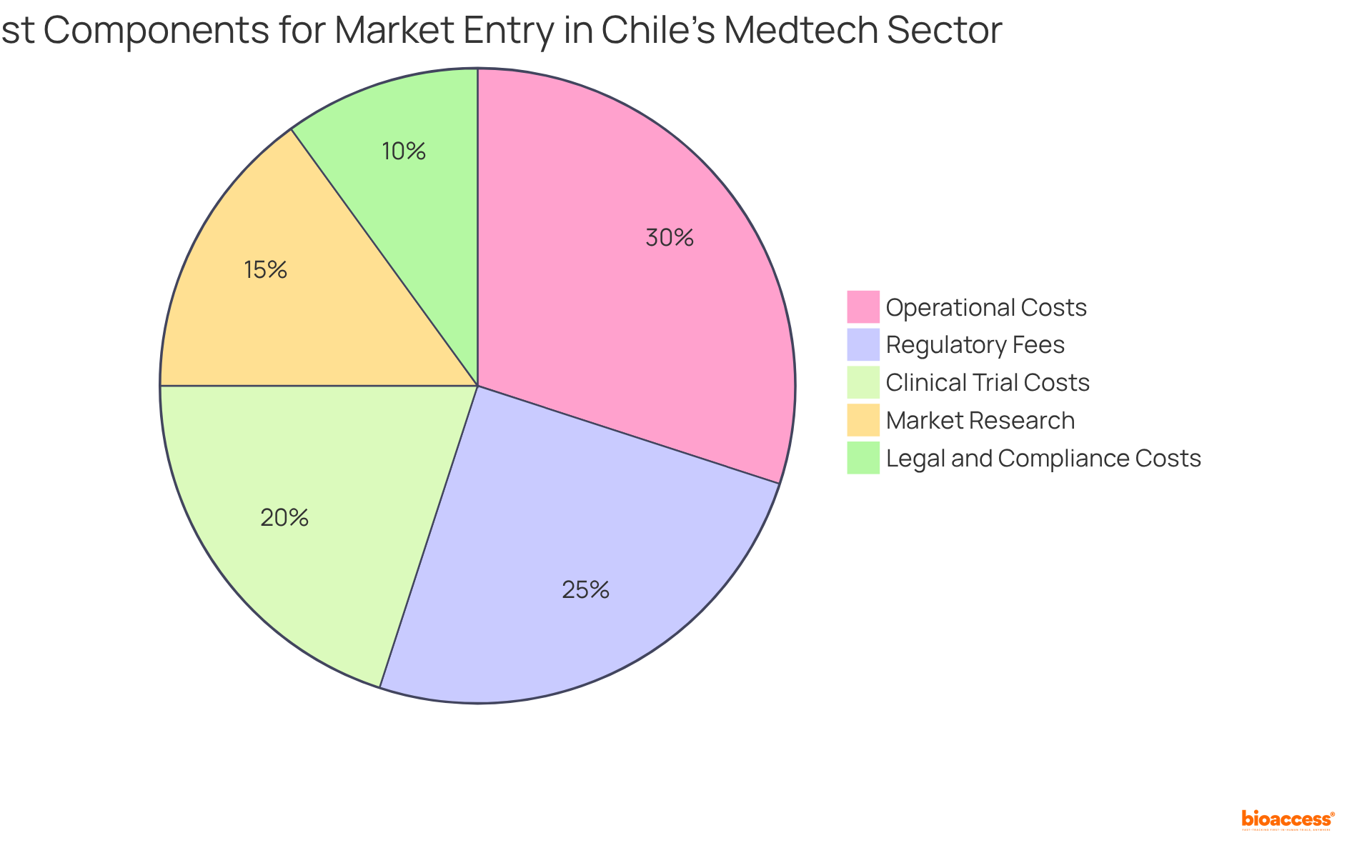
When evaluating market entry feasibility costs in Chile, Medtech innovators must consider several critical components:
Regulatory Fees: The costs associated with obtaining necessary approvals from the Chilean health authorities, particularly the Instituto de Salud Pública (ISP), vary based on the medical device type and classification. For example, sanitary registration fees for general medical devices are approximately CLP 160,027 + VAT, while in vitro diagnostic devices incur fees of CLP 471,382 + VAT. Additionally, modifications to registration details cost CLP 66,977 + VAT.
Operational Costs: This encompasses expenses related to establishing operations, such as hiring local staff, renting facilities, and logistics. The cost of living and wage standards in Chile can significantly impact these expenses, necessitating careful budgeting.
Clinical Trial Costs: Should clinical trials be necessary, it is essential to budget for patient recruitment, site management, and data collection. Conducting trials in Chile can offer substantial cost advantages, with operational expenses potentially reduced by up to 30% compared to North America and Europe, while also allowing for faster patient enrollment.
Market Research: Gaining insights into local demand, competition, and pricing strategies is crucial. This may involve hiring local consultants or firms specializing in trend analysis to ensure informed decision-making.
Legal and Compliance Costs: Engaging legal expertise to navigate the regulatory landscape and ensure compliance with local laws can incur additional costs, which should be factored into the overall budget.
By thoroughly analyzing these components, technology firms can develop a comprehensive budget that addresses the market entry feasibility cost in Chile, ensuring preparedness for the financial commitments involved in entering the Chilean economy.
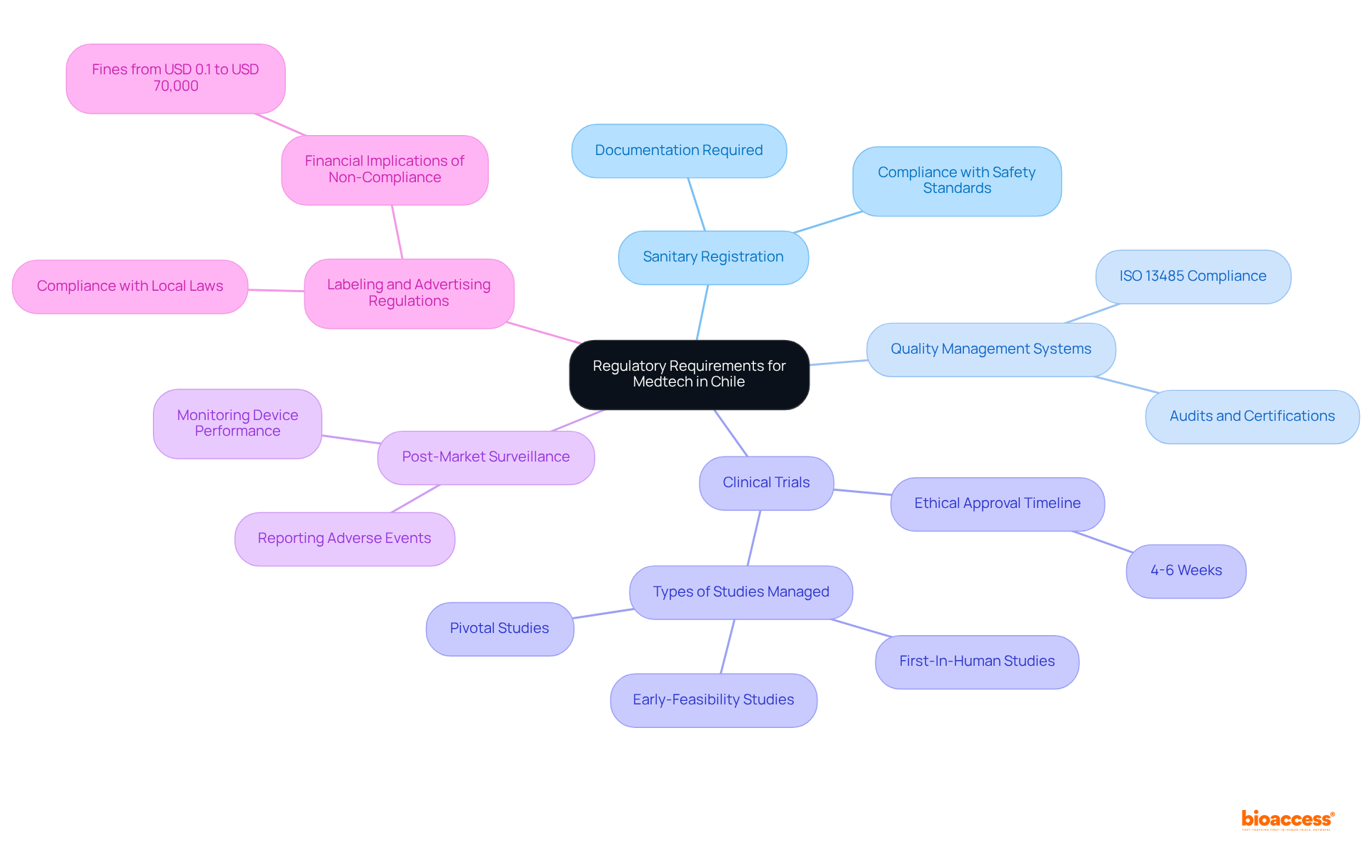
Navigating the regulatory landscape in Chile is essential for Medtech innovators. Key regulatory requirements include:
Sanitary Registration: All medical devices must be registered with the Instituto de Salud Pública (ISP). This process requires detailed documentation, including clinical data, manufacturing processes, and labeling information. The sanitary registration procedure is intended to guarantee that products comply with safety and efficacy standards prior to being available for sale.
Quality Management Systems: Compliance with international quality standards, such as ISO 13485, is mandatory. Companies must demonstrate adherence through audits and certifications from recognized bodies, ensuring that their design and manufacturing processes meet stringent quality requirements.
Clinical Trials: If clinical trials are necessary, they must comply with local regulations, including obtaining ethical approval from relevant committees. The duration and complexity of this process can vary significantly based on the device type and its intended use. With bioaccess®, ethical approvals usually require 4-6 weeks, and their expertise enables patient enrollment to be accomplished 50% quicker than conventional sectors, leading to considerable time and cost savings. bioaccess® specializes in managing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF).
Post-Market Surveillance: After a device is launched, companies are required to monitor its performance and report any adverse events to the ISP. This continuous compliance is essential for preserving authorization and ensuring patient safety.
Labeling and Advertising Regulations: All promotional materials and product labeling must adhere to local laws, ensuring that claims are substantiated and that information is clear and accurate. The Sanitary Code mandates that safety and proper usage warnings be included in the labeling of life sciences products, which is vital for compliance and risk management. Fines for sanitary infringements can range from USD 0.1 to USD 70,000, highlighting the financial implications of non-compliance.
By thoroughly understanding these regulatory requirements, medical technology firms can effectively assess the market entry feasibility cost in Chile and mitigate potential compliance risks, ultimately enhancing their chances for successful commercialization. As stated by the Ministerio de Salud, "This regulatory update aims to enhance patient safety by enforcing strict compliance with quality standards for these devices.
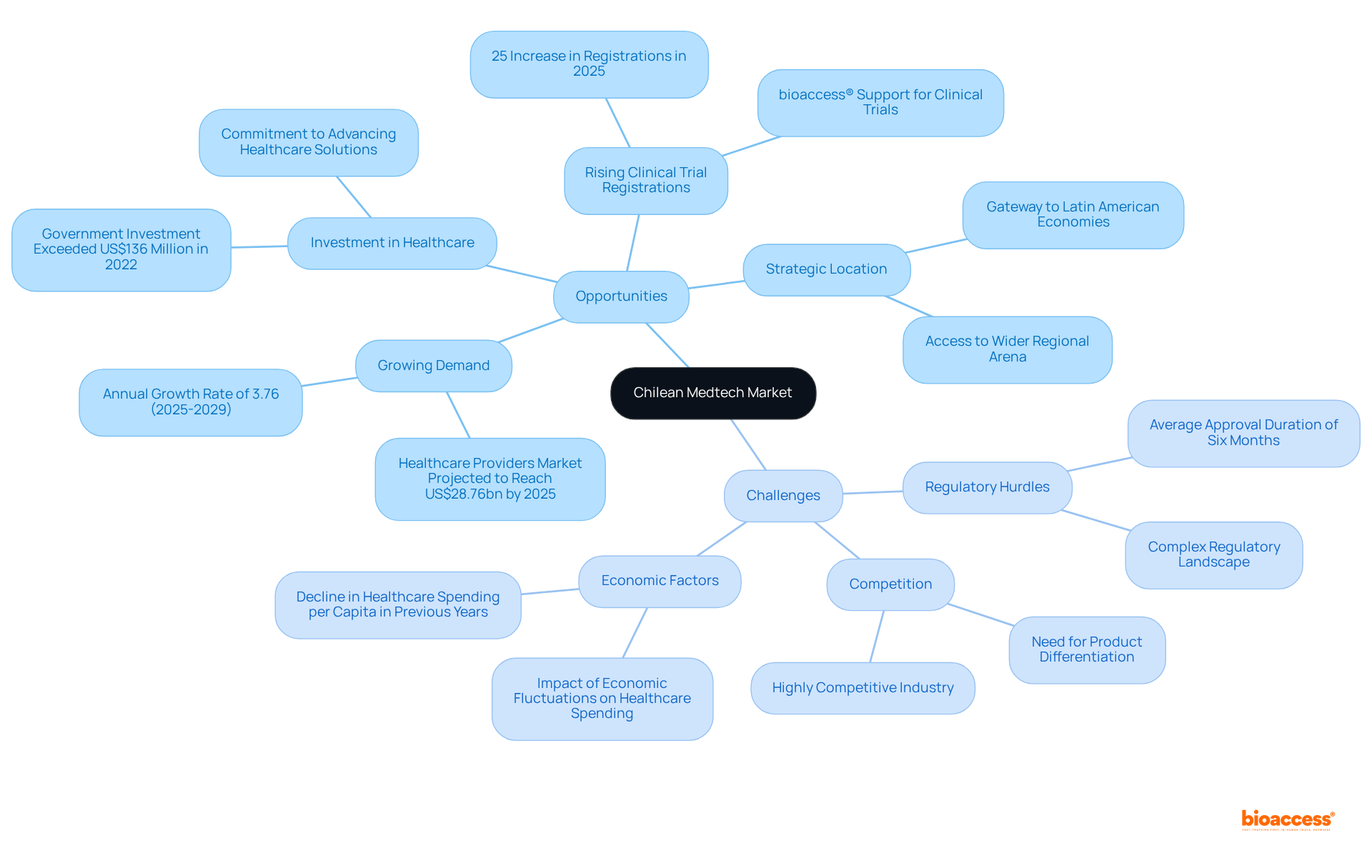
The Chilean Medtech market presents a dynamic landscape rich with opportunities and challenges for innovators.
Opportunities:
Challenges:
By recognizing these opportunities and challenges, medical technology innovators can craft targeted strategies that leverage their strengths while effectively addressing the market entry feasibility cost in Chile. Additionally, the impact of Medtech clinical studies on local economies, including job creation and healthcare improvement, underscores the importance of these initiatives.
Evaluating the market entry feasibility cost in Chile is vital for Medtech innovators aiming to leverage the country's robust healthcare landscape. This analysis not only highlights the growth potential within the sector but also emphasizes the necessity of understanding regulatory requirements, operational expenses, and strategic partnerships to ensure a successful entry.
Key insights illustrate that Chile's healthcare system presents a favorable environment for new medical technologies, driven by rising consumer demand and government support. Innovators must navigate various cost components, including regulatory fees, operational expenses, and clinical trial costs, while also addressing the challenges posed by competition and economic fluctuations. Engaging with local distributors and utilizing comprehensive clinical trial management services can significantly enhance the likelihood of success in this dynamic market.
Ultimately, the Medtech sector in Chile offers a wealth of opportunities for innovators willing to invest the necessary time and resources to comprehend the market landscape. By meticulously assessing market entry feasibility costs and strategically positioning themselves, companies can contribute to the advancement of healthcare in Chile while expanding their reach across the broader Latin American market. Embracing these insights and taking proactive steps can lead to impactful innovations that improve patient outcomes and drive industry growth.
What is market entry feasibility in the context of Chile?
Market entry feasibility in Chile involves evaluating the viability of entering a new sector by considering regulatory requirements, demand, competition, and operational costs, particularly in the robust healthcare system.
What is the projected volume of Chile's healthcare sector by 2025?
By 2025, Chile's healthcare sector is projected to reach approximately US$2.49 billion, driven by increasing consumer demand for advanced medical technologies and a stable economic environment.
How does Chile's healthcare system support Medtech innovators?
Chile's healthcare system, which includes both public and private providers, facilitates access for Medtech innovators through government policies that promote advanced medical devices, including subsidies and support for research and development.
What are the regulatory advantages for entering the Chilean market?
Chile offers relatively low import tariffs and requires certification under international standards, such as EU CE marking and U.S. FDA approval, making it an attractive market for medical technology firms.
What factors enhance patient recruitment in Chile?
The potential for patient recruitment in Chile is strengthened by a well-educated workforce and a robust research and development ecosystem, including universities and research institutes engaged in medical technology.
What trends are influencing the demand for medical technologies in Chile?
There is a growing trend towards digital health solutions, such as telemedicine and wearable devices, which are increasingly in demand among consumers in Chile.
What strategies should medical technology firms consider for entering the Chilean market?
Firms should consider forming partnerships with local distributors who have strong networks in both public and private sectors to enhance industry presence and ensure efficient after-sale service.
How can clinical trial management services assist in market entry?
Leveraging comprehensive clinical trial management services, such as those offered by bioaccess®, can increase the likelihood of successful industry entry by providing support for Pilot Studies and Post-Market Clinical Follow-Up Studies.