


MDUFA, or the Medical Device User Fee Amendments, is pivotal in clinical research as it empowers the FDA to collect user fees that enhance its capacity to evaluate medical device applications more efficiently. This framework has significantly curtailed review times for submissions, facilitating quicker access to innovative healthcare technologies and ultimately improving patient outcomes. Such developments underscore MDUFA's positive impact on regulatory processes and clinical research practices, reinforcing its essential role in advancing healthcare.
The Medical Device User Fee Amendments (MDUFA) stand as a pivotal element in the regulatory landscape, profoundly shaping the pathway for medical devices to enter the market. By empowering the FDA to collect user fees, MDUFA bolsters the agency's capacity to accelerate the review process, thereby enhancing patient access to groundbreaking healthcare technologies. Yet, as the medical technology landscape evolves, one must ponder: how adeptly can MDUFA adjust to address the forthcoming challenges in clinical research and regulatory requirements?
The User Fee Amendments for Medical Equipment stand as a pivotal framework established between the U.S. Food and Drug Administration (FDA) and the medical equipment sector. This initiative empowers the FDA to collect user fees from producers, which are vital for enhancing the agency's capacity to evaluate healthcare equipment applications with greater efficiency and effectiveness. Since its inception in 2002, this initiative has been reauthorized every five years, with the current iteration, referred to as mdufa V, set to remain in effect until September 2027.
This legislation is instrumental in expediting the assessment process for medical devices under mdufa, significantly improving patient access to innovative technologies. Under the new regulatory framework, for instance, 95% of 510(k) submissions are expected to experience meaningful interaction within 60 days and a resolution within 90 days. This development has contributed to a downward trend in average turnaround times (TaT) for 510(k) submissions, which have decreased since 2018, underscoring the positive impact of the legislation on regulatory efficiency.
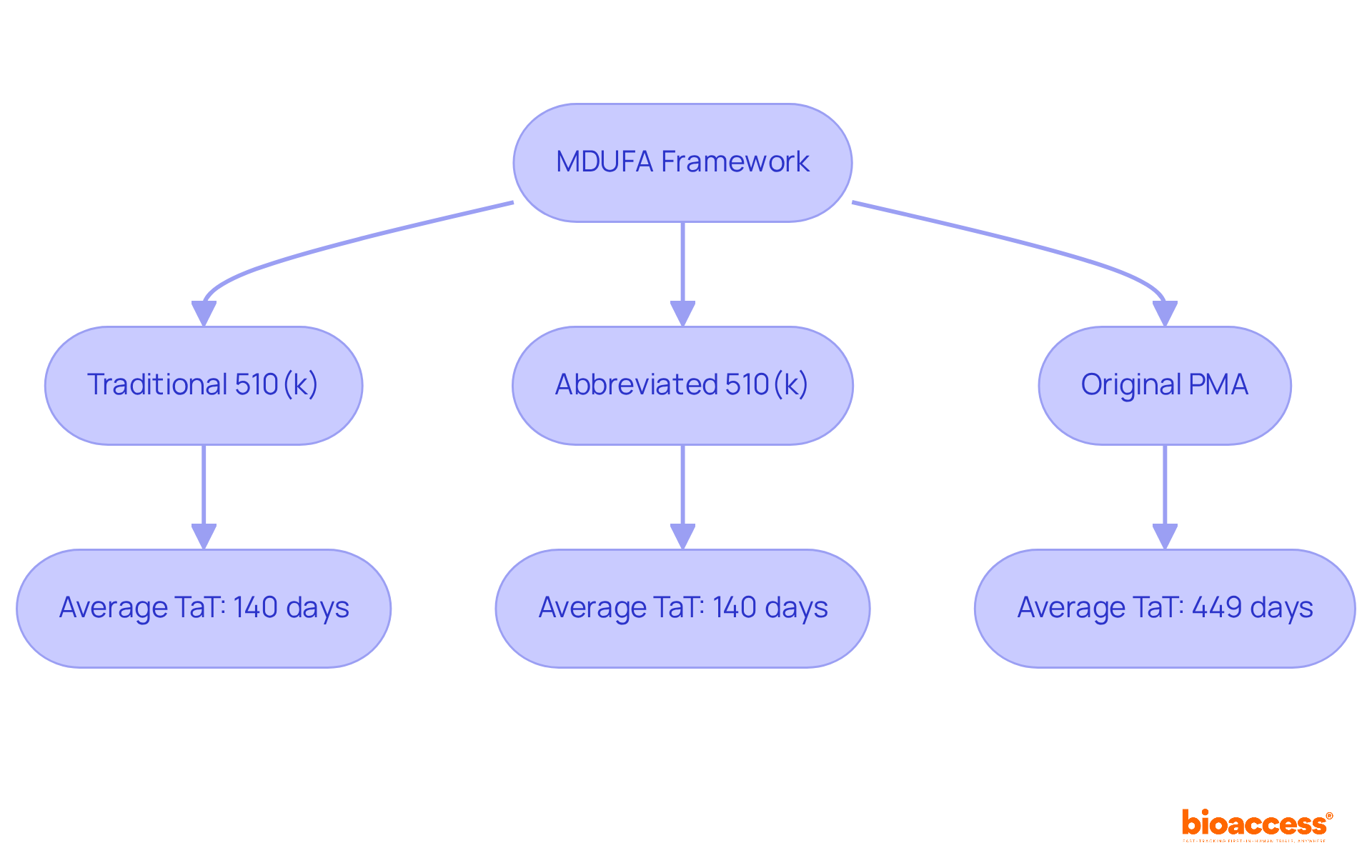
Real-world examples showcase the program's effectiveness in enhancing access to groundbreaking healthcare technologies. The average TaT for Traditional and Abbreviated 510(k) submissions is approximately 140 days, while Original PMAs average 449 days. This trend not only highlights the FDA's commitment to fostering innovation but also illustrates the critical role of the act in clinical research, particularly as we approach 2025. By streamlining the approval process, the act ensures that transformative healthcare products reach the market more swiftly, ultimately benefiting both patients and providers.
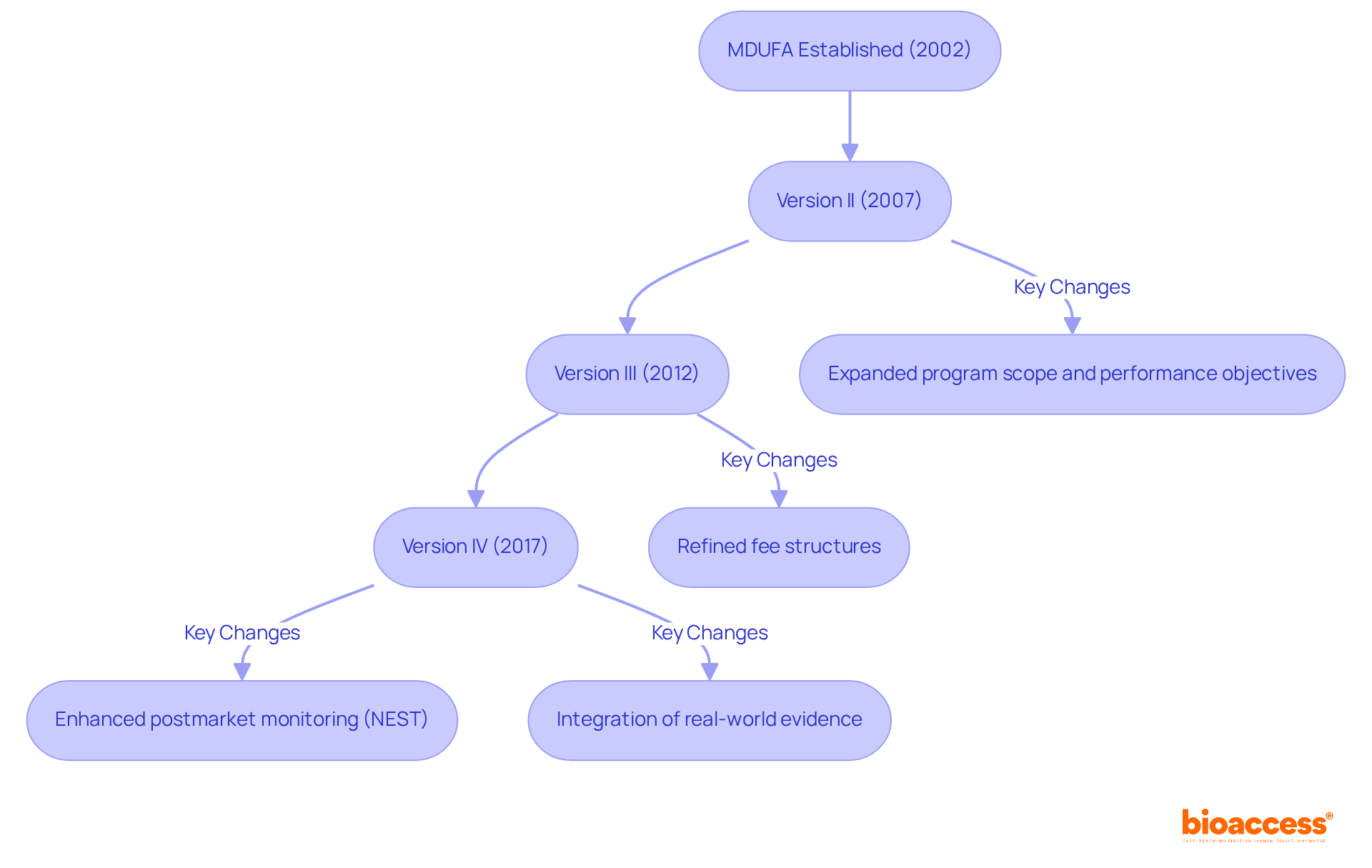
The Medical Equipment User Fee Amendments were established in response to growing concerns regarding the FDA's ability to effectively evaluate an increasing number of device applications. Initially enacted in 2002, this user fee program was designed to enhance the FDA's resources through user fees, thereby improving the agency's capability to adhere to established review timelines. Subsequent reauthorizations—version II in 2007, version III in 2012, and version IV in 2017—have progressively expanded the program's scope, introduced new performance objectives, and refined fee structures to align with the evolving healthcare technology landscape. Each iteration has focused on boosting the efficiency and predictability of the review process, ensuring that the FDA can effectively keep pace with advancements in the healthcare equipment sector. Notably, the fourth iteration of the Medical Device User Fee Amendments not only facilitates timely evaluations but also enhances postmarket monitoring through the National Evaluation System for health Technology (NEST), underscoring a commitment to integrating real-world evidence into regulatory practices. This evolution underscores the critical role of the act in fostering the advancement of healthcare tools while addressing the challenges of regulatory compliance in a rapidly changing environment.
In Colombia, the National Food and Drug Surveillance Institute (INVIMA) plays a vital role in regulating health equipment, ensuring compliance with health standards and overseeing the marketing and production of health products. As a Level 4 health authority recognized by the Pan American Health Organization/World Health Organization, INVIMA's regulatory framework is essential for maintaining the safety, efficacy, and quality of medical devices in Colombia, similar to the objectives of MDUFA in the U.S.
The components of mdufa encompass several critical elements, including the collection of user fees, performance goals, and established review timelines. The categorization of user fees includes various types, such as application fees for premarket approvals (PMAs) and annual establishment registration fees. Notably, the third iteration of the user fee agreement permitted the FDA to gather $595 million from the industry, underscoring the financial influence of user fees on FDA activities.
The FDA is committed to specific performance goals, which include:
For instance, under this third iteration of the user fee agreement, the FDA authorized 95% of PMAs and 85% of 510(k)s accepted for review, significantly accelerating the marketing application process. Furthermore, user fees accounted for 42% of the FDA's total program level in FY2016, highlighting the growing reliance on these fees.
Provisions from the legislation also promote stakeholder involvement, illustrated by the public meeting conducted on November 2, 2016, which enabled industry representatives to offer insights on the program's effectiveness and possible enhancements. This organized approach allows the FDA to distribute resources effectively while maintaining a high level of review quality, ultimately benefiting both the industry and patients awaiting new health products.

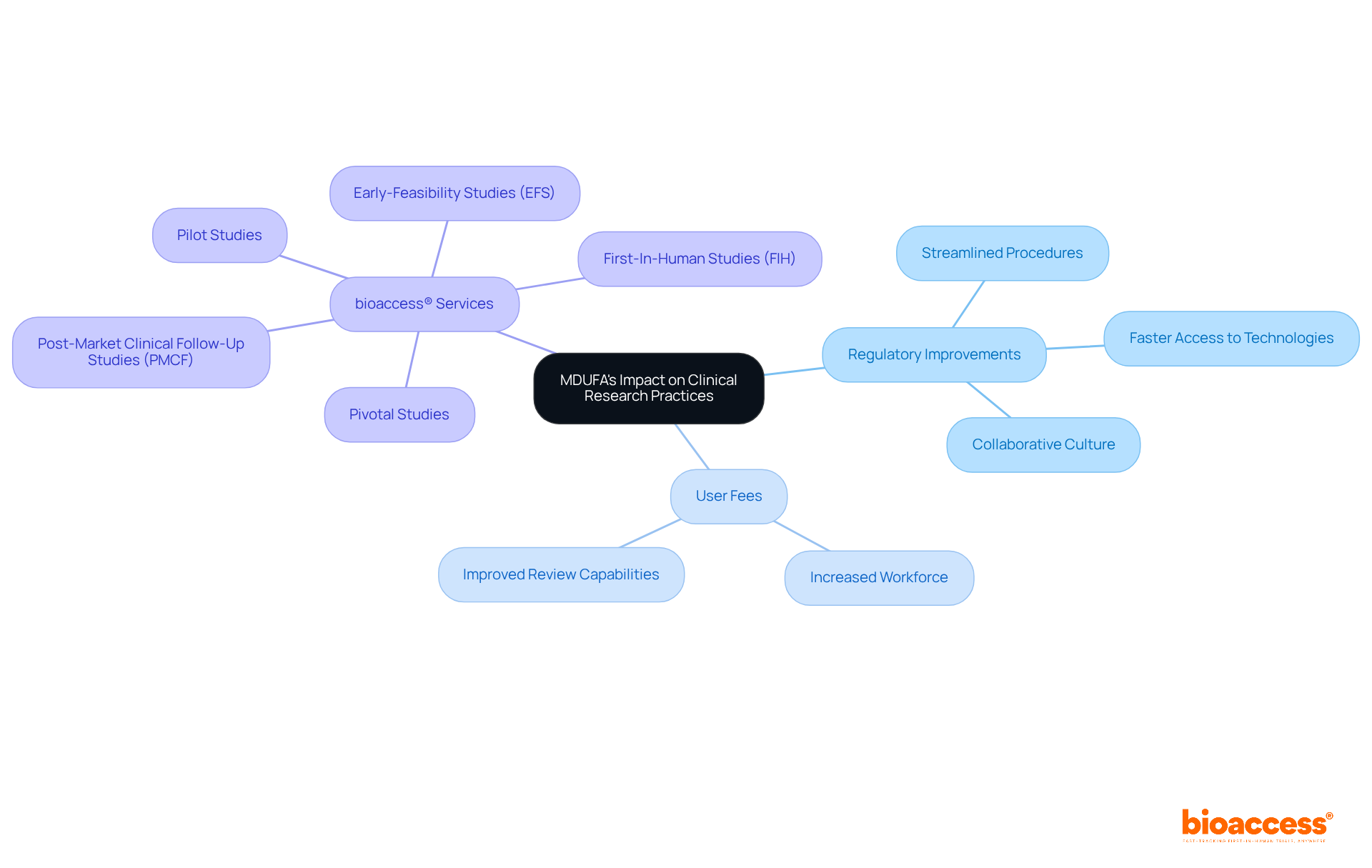
The program has significantly transformed clinical research methods by streamlining the regulatory procedures for medical equipment. By reducing review durations, it enables faster access to innovative technologies, ultimately enhancing patient outcomes. Notably, the introduction of user fees has allowed the FDA to increase its workforce and improve review capabilities, resulting in swifter decisions on application submissions. Furthermore, MDUFA has fostered a collaborative culture between the FDA and the device industry, promoting ongoing dialogue about regulatory challenges and innovations. This collaborative environment is vital for keeping pace with the rapid advancements in medical technology and ensuring that regulatory processes align with industry needs.
In this context, bioaccess® provides comprehensive clinical trial management services, drawing on over 20 years of expertise in Medtech to effectively manage:
By emphasizing specialized knowledge and adaptability, bioaccess® not only enhances the efficiency of clinical trials but also contributes to local economies through job creation, economic growth, and improved healthcare outcomes.
The Medical Device User Fee Amendments (MDUFA) serve as a pivotal mechanism in enhancing the efficiency of medical device regulation, profoundly impacting the realm of clinical research. By instituting a system where user fees finance the FDA's capacity to assess medical equipment applications, MDUFA has streamlined the approval process, ensuring that innovative healthcare technologies can reach patients more swiftly and effectively.
Since its inception in 2002, MDUFA has continually evolved to meet the dynamic demands of the healthcare technology sector. Each iteration has introduced new performance goals and refined fee structures, resulting in significantly reduced turnaround times for device submissions. This act has not only accelerated the pace of evaluations but also fostered collaboration between the FDA and industry stakeholders, cultivating an environment that promotes innovation and responsiveness to regulatory challenges.
As the healthcare landscape continues to transform, the significance of MDUFA remains paramount. Its role in expediting the approval process for medical devices is essential for enhancing patient access to new technologies and improving overall healthcare outcomes. Embracing the principles of MDUFA will be crucial for stakeholders in the medical device industry as they navigate the complexities of regulatory compliance and endeavor to introduce transformative products to market.
What is MDUFA?
MDUFA stands for the User Fee Amendments for Medical Equipment, a framework established between the U.S. Food and Drug Administration (FDA) and the medical equipment sector that allows the FDA to collect user fees from producers to enhance its capacity to evaluate healthcare equipment applications.
When was MDUFA first established and how often is it reauthorized?
MDUFA was first established in 2002 and has been reauthorized every five years. The current iteration, known as MDUFA V, is set to remain in effect until September 2027.
How does MDUFA impact the assessment process for medical devices?
MDUFA significantly improves the assessment process for medical devices by expediting evaluations, ensuring that 95% of 510(k) submissions have meaningful interactions within 60 days and resolutions within 90 days.
What are the average turnaround times (TaT) for 510(k) submissions under MDUFA?
The average turnaround time for Traditional and Abbreviated 510(k) submissions is approximately 140 days, while Original PMAs average 449 days.
What benefits does MDUFA provide to patients and healthcare providers?
MDUFA benefits patients and healthcare providers by streamlining the approval process, allowing transformative healthcare products to reach the market more swiftly, thus improving patient access to innovative technologies.
How has MDUFA affected regulatory efficiency since its inception?
Since the inception of MDUFA, there has been a downward trend in average turnaround times for 510(k) submissions, indicating a positive impact on regulatory efficiency and the FDA's commitment to fostering innovation in healthcare technologies.