

This article defines what constitutes a medical device according to the FDA, emphasizing its extensive scope and the critical importance of compliance for manufacturers and researchers. It outlines the FDA's classification of medical devices into three risk-based categories, detailing the regulatory pathways for market entry. Understanding these definitions and classifications is essential for effectively navigating the approval process, making it a significant focus for those involved in clinical research.
Understanding the FDA's definition of medical devices is essential for anyone engaged in the healthcare industry, including manufacturers and clinical researchers. This comprehensive definition covers a broad spectrum of products, each accompanied by specific regulatory requirements and classifications that dictate their market entry pathway.
However, the intricate nature of these regulations can be overwhelming, particularly in light of recent changes that influence approval processes and compliance obligations. Manufacturers face significant challenges in ensuring their devices align with FDA standards.
How can they adeptly navigate the regulatory landscape to secure successful market entry?
According to the medical device definition by FDA, a medical instrument is any tool, apparatus, implement, machine, contrivance, implant, in vitro reagent, or similar article intended for use in the diagnosis, cure, mitigation, treatment, or prevention of disease. This expansive definition encompasses a wide array of products, ranging from simple tongue depressors to advanced programmable pacemakers.
Understanding the medical device definition by FDA is vital for manufacturers and researchers, as it ensures compliance with regulatory requirements. The intended use and indications for use are essential in determining whether a product qualifies as a medical instrument, as stipulated in Section 201(h) of the Federal Food, Drug, and Cosmetic Act.
Recent updates to FDA medical equipment criteria have highlighted the need for clarity in classification. Notably, approximately 85 percent of 510(k) applications received a Substantially Equivalent decision, illustrating a robust pathway for many products. However, it is important to note that nearly 32 percent of submissions failed the initial acceptance for review check in the year leading up to September 2022, emphasizing the necessity of thorough preparation and a deep understanding of FDA criteria.
Examples of medical equipment classified under these criteria include:
This showcases the diverse applications of medical technology in contemporary healthcare.
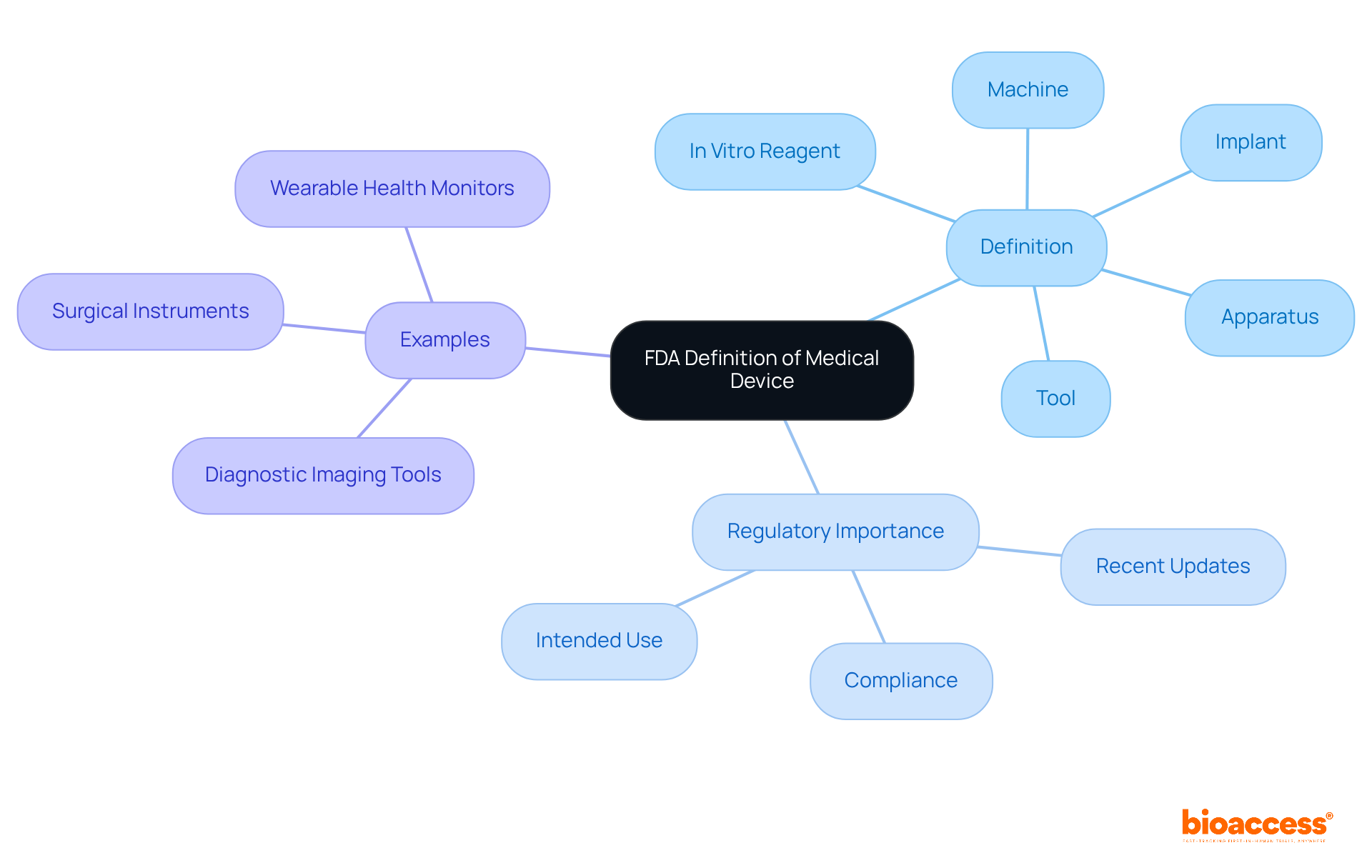
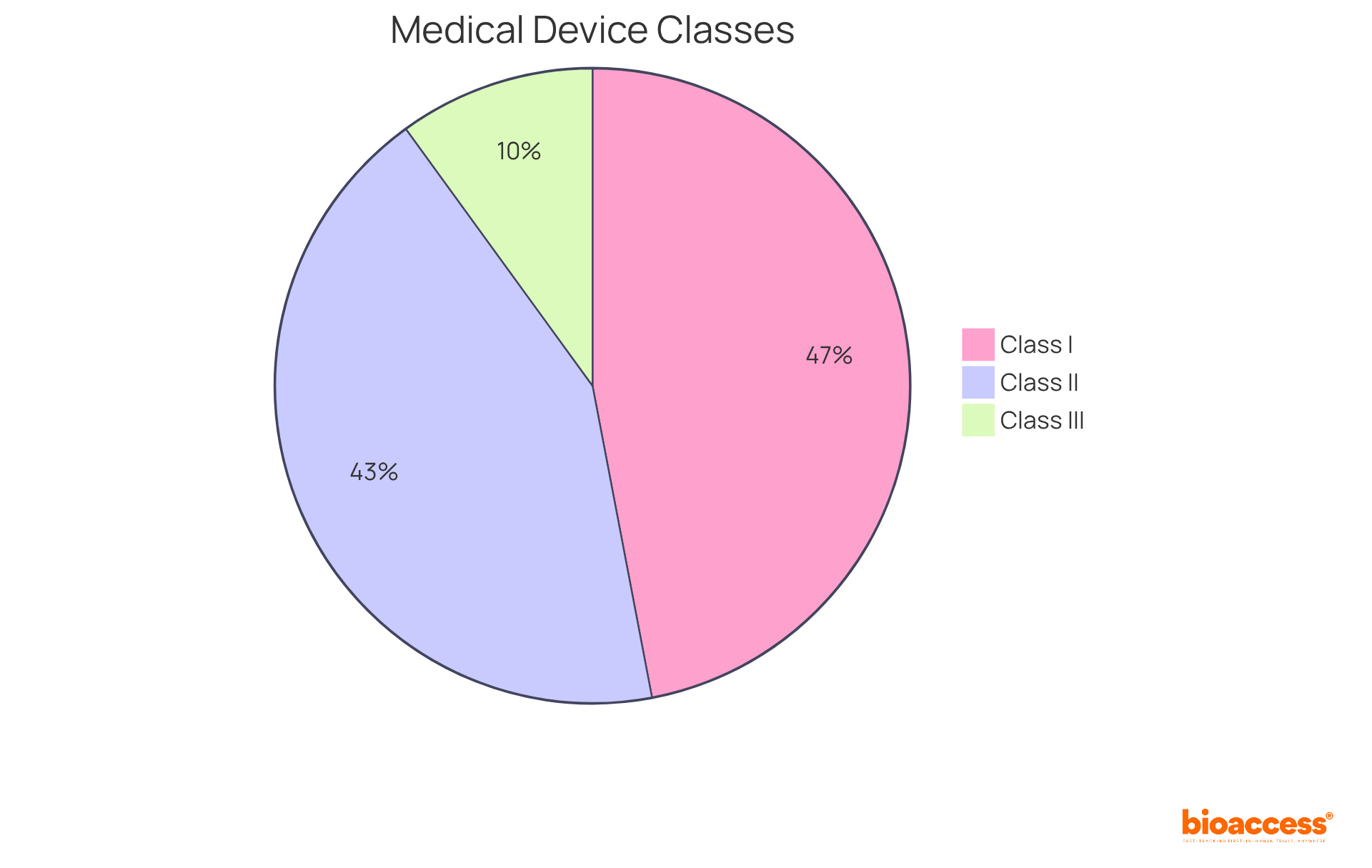
The FDA classifies medical devices into three categories based on their associated risk levels:
Class I: These devices are classified as low risk and are subject to minimal regulatory control. Examples include bandages and handheld surgical instruments. Notably, the majority of Class I products are exempt from premarket notification, comprising approximately 47% of all approved items in the market.
Class II: Representing around 43% of all medical products, Class II items present moderate risk and typically require a 510(k) premarket notification. This process demonstrates that the device is substantially equivalent to a legally marketed counterpart. Common examples include infusion pumps and surgical drapes, with successful submissions in this category facilitating market entry for innovative solutions.
Class III: These high-risk products require premarket approval (PMA) to confirm their safety and effectiveness. Examples encompass implantable pacemakers and breast implants. The PMA process is stringent, often involving extensive clinical trials and data collection to ensure adherence to safety standards.
Recent changes in FDA regulations have highlighted the significance of human factors engineering (HFE) for certain risk-based classes, thereby enhancing the focus on user-related risks. Understanding these classifications is crucial for navigating the regulatory landscape effectively, as they dictate the level of oversight and the compliance measures required for various categories of medical equipment, which are outlined in the medical device definition by FDA.
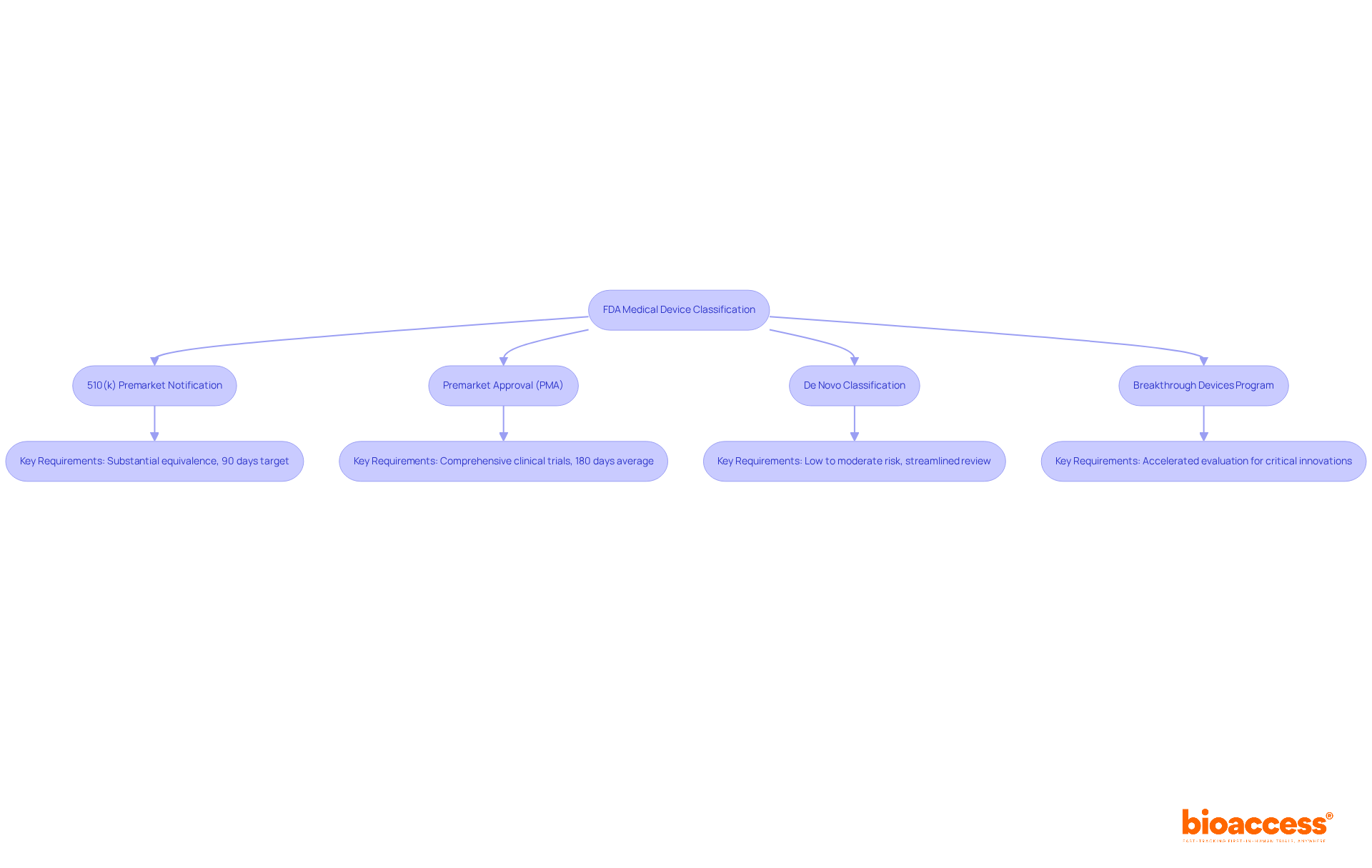
Once classified under the medical device definition by FDA, medical device manufacturers must navigate specific pathways to achieve market entry.
510(k) Premarket Notification: For Class II products, manufacturers are required to submit a 510(k) application to demonstrate substantial equivalence to an existing product. While the FDA aims to provide a decision within 90 days, the average time for a 510(k) decision often extends to 140-200 days, with only a small fraction achieving a decision within the target timeframe.
Premarket Approval (PMA): Class III products undergo a more rigorous PMA process, necessitating comprehensive clinical trials to establish safety and effectiveness. This pathway can span several months to years, with average PMA application processing durations reaching up to 180 days for standard evaluations and even longer for intricate products. The documentation required encompasses clinical data, design specifications, and safety assessments, making it essential for manufacturers to prepare thoroughly.
De Novo Classification: This pathway is intended for new products categorized as low to moderate risk but lacking a predicate. It allows for a streamlined review process, facilitating quicker market access for innovative solutions.
Breakthrough Devices Program: This initiative accelerates the creation and evaluation of instruments that provide substantial benefits compared to current alternatives for life-threatening or irreversibly debilitating conditions. By prioritizing these devices, the FDA aims to enhance patient access to critical innovations.
Grasping these pathways is crucial for producers to effectively devise their regulatory strategies and maneuver through the intricacies of the approval process as defined by the medical device definition by FDA. With over 20 years of experience in Medtech, bioaccess offers comprehensive clinical trial management services, including Early-Feasibility Studies, First-In-Human Studies, and Post-Market Clinical Follow-Up Studies, specifically tailored for the Latin American market. This expertise allows producers to streamline their submissions and improve their chances of success. Starting October 1, 2023, the FDA mandates digital submissions via the eSTAR platform, which will further streamline the application process.
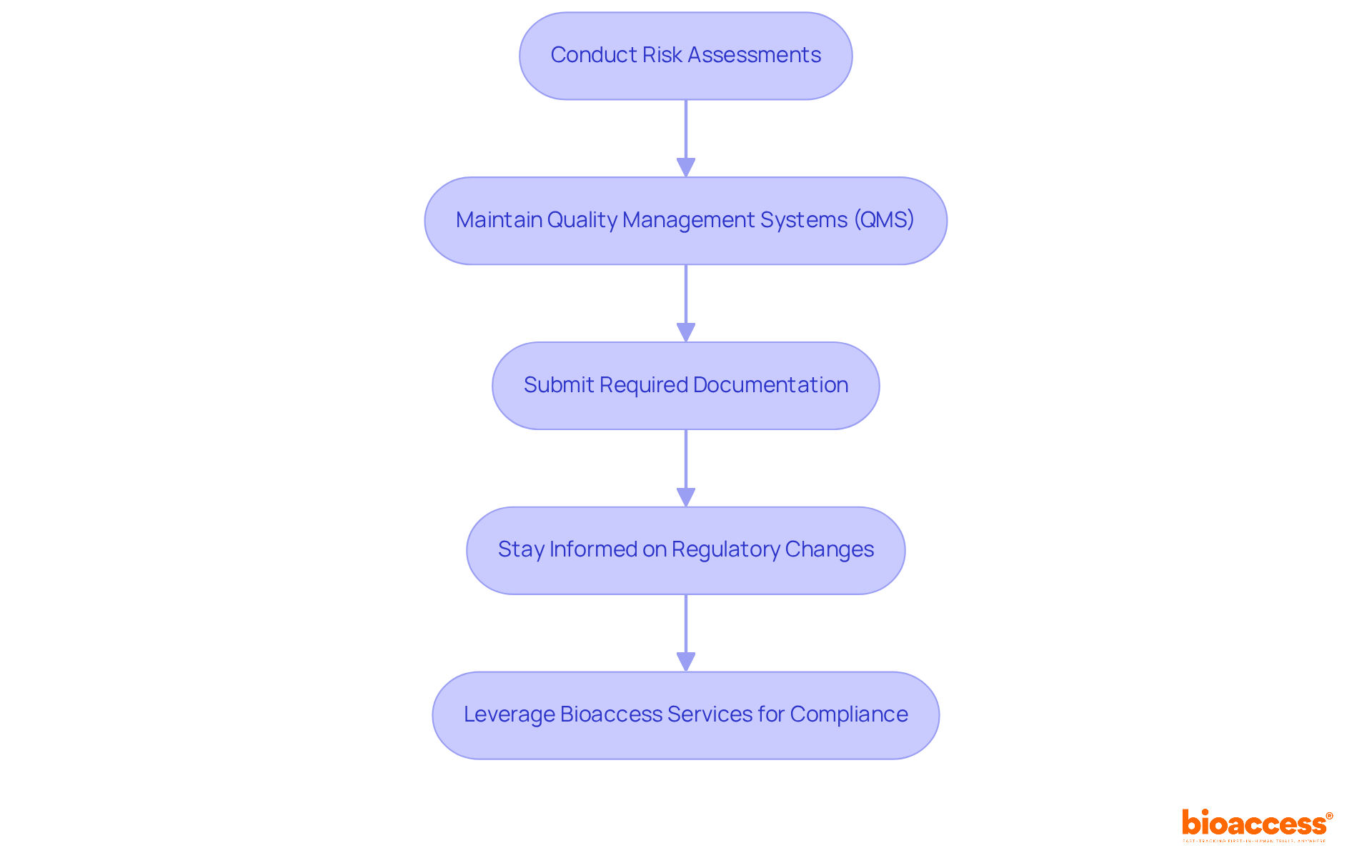
To ensure compliance with FDA regulations, manufacturers must take several critical steps:
Conduct Risk Assessments: Regularly evaluate potential risks associated with the device, implementing appropriate controls to mitigate these risks. This proactive approach is crucial, as every clinical investigation presents unique challenges arising from both the product and the study protocol.
Maintain Quality Management Systems (QMS): Establish and uphold a robust QMS that is in compliance with the medical device definition by FDA's Quality System Regulation (QSR). A well-implemented QMS not only ensures consistent product quality but also enhances compliance. Studies indicate that organizations with mature quality management systems achieve a 92% on-time delivery rate compared to 74% for those without.
Submit Required Documentation: Prepare and submit all necessary documentation, including premarket submissions, labeling, and post-market surveillance reports. This documentation is crucial for demonstrating compliance with the medical device definition by FDA and ensuring that all regulatory requirements are met.
Stay Informed on Regulatory Changes: Continuously monitor updates to the medical device definition by FDA and other regulations and guidance documents to maintain ongoing compliance. With the FDA's recent amendments to the Quality Management System Regulations (QMSR), producers must adapt their practices to align with these evolving standards.
In addition to these obligations, bioaccess offers comprehensive clinical trial management services, including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting. For instance, bioaccess provides thorough review and feedback on study documents to ensure compliance with country requirements, which is critical for successful market entry. By leveraging these services, manufacturers can effectively mitigate risks and enhance their chances of successful market entry, ultimately contributing to improved patient safety and product efficacy. Katherine Ruiz, an expert in regulatory affairs for medical devices and in vitro diagnostics in Colombia, leads these initiatives, ensuring that bioaccess remains at the forefront of innovation and regulatory excellence in Latin America.
Understanding the definition of medical devices as outlined by the FDA is crucial for navigating the complex landscape of clinical research and regulatory compliance. This definition encompasses a wide range of products, from simple tools to sophisticated technologies, all aimed at improving health outcomes. By grasping the nuances of this definition, manufacturers and researchers can ensure that their products meet the necessary regulatory standards, ultimately facilitating safer and more effective medical innovations.
Key points highlighted throughout the article include:
In light of these insights, it becomes evident that a deep understanding of the FDA's medical device criteria is not just beneficial but essential for stakeholders in the healthcare sector. As the landscape of medical technology continues to evolve, staying abreast of regulatory obligations and approval processes will empower manufacturers to innovate while ensuring patient safety and product efficacy. Engaging with these guidelines and leveraging available resources can pave the way for successful advancements in medical technology, ultimately enhancing the quality of healthcare delivery.
What is the FDA's definition of a medical device?
According to the FDA, a medical device is any tool, apparatus, implement, machine, contrivance, implant, in vitro reagent, or similar article intended for use in the diagnosis, cure, mitigation, treatment, or prevention of disease.
Why is understanding the medical device definition by the FDA important for manufacturers and researchers?
Understanding the medical device definition is vital for manufacturers and researchers as it ensures compliance with regulatory requirements.
What factors determine whether a product qualifies as a medical device?
The intended use and indications for use are essential factors in determining whether a product qualifies as a medical device, as outlined in Section 201(h) of the Federal Food, Drug, and Cosmetic Act.
What recent trends have been observed in FDA medical device applications?
Recent updates highlight that approximately 85 percent of 510(k) applications received a Substantially Equivalent decision, indicating a robust pathway for many products. However, nearly 32 percent of submissions failed the initial acceptance for review check in the year leading up to September 2022.
Can you provide examples of medical devices classified under FDA criteria?
Examples of medical devices include diagnostic imaging tools, surgical instruments, and wearable health monitors, showcasing the diverse applications of medical technology in healthcare.