

The article examines the meaning and significance of medical devices, underscoring their diverse applications in diagnosing, preventing, monitoring, and treating health conditions. It asserts the critical role of regulatory frameworks and intended purposes in ensuring the safety and efficacy of these devices. Supported by compelling statistics and advancements in technology, the discussion highlights their transformative impact on patient care, inviting readers to recognize the importance of these innovations in clinical practice.
Understanding the landscape of medical devices unveils a complex interplay of technology, regulation, and patient care that is essential for modern healthcare. These instruments, which range from simple bandages to sophisticated robotic systems, are pivotal in diagnosing, treating, and monitoring health conditions.
As advancements continue to reshape the industry, a pressing question emerges: how can stakeholders effectively navigate the intricate regulatory frameworks and classifications governing medical devices to ensure safety, efficacy, and accessibility? This inquiry is not merely academic; it is a critical consideration for all involved in clinical research and healthcare delivery.
A healthcare instrument encompasses a diverse array of items, including tools, equipment, machines, implants, in vitro reagents, and software, all tailored for health-related purposes. This definition spans from basic implements like bandages to sophisticated imaging systems, highlighting the multifaceted nature of healthcare instruments. Their paramount significance lies in their capacity to diagnose, prevent, monitor, and treat various health conditions, thereby serving as a cornerstone of modern healthcare.
Recent advancements in healthcare instruments, particularly in 2025, have focused on enhancing accessibility and accuracy. For instance, innovations in robotic systems are facilitating the rise of minimally invasive procedures, which markedly improve patient outcomes by shortening recovery times and minimizing complications. The orthopedic robotics market alone is anticipated to surpass $3.5 billion by 2030, indicating a compound annual growth rate exceeding 10%.
The practical applications of healthcare instruments vividly demonstrate their transformative influence on patient care. Robotic-assisted surgeries, for example, have resulted in enhanced implant longevity and a reduction in corrective surgeries, thereby underscoring the efficacy of these technologies in improving surgical precision. Additionally, wearable health devices, such as smartwatches and biosensors, are revolutionizing real-time health monitoring, enabling proactive management of chronic conditions and reducing hospital visits.
Expert opinions underscore the critical role of healthcare instruments in contemporary medicine. As the healthcare landscape evolves toward more personalized and anticipatory approaches, the integration of AI and machine learning into health technologies is becoming increasingly vital. These innovations not only bolster diagnostic accuracy but also facilitate remote patient monitoring, which can avert emergency visits and hospital readmissions, ultimately conserving billions in healthcare expenditures.
Statistics further illustrate the impact of healthcare tools on health outcomes. Notably, a significant portion of healthcare instruments withdrawn due to serious health concerns had gained approval through the less stringent 510(k) process, underscoring the need for rigorous oversight. Moreover, research indicates that only 16% of randomly selected implants had publicly accessible scientific evidence supporting their safety and effectiveness, emphasizing the importance of transparency in the healthcare equipment sector.
Understanding the medical devices meaning and its significance is crucial for stakeholders within the health sector, as it informs compliance with regulations, product development, and market access strategies, ultimately leading to improved patient care and health outcomes.
The regulatory frameworks overseeing the medical devices meaning exhibit considerable variation worldwide. In the United States, the Food and Drug Administration (FDA) organizes instruments into three categories according to risk:
Category I items, which pose minimal danger, can often self-declare compliance, while Category II items require premarket submissions and post-market surveillance. Group III products encounter the most rigorous standards, requiring thorough clinical assessments and continuous oversight to guarantee safety and effectiveness.
Conversely, the European Union's Medical Device Regulation (MDR) employs a similar classification system but places a stronger emphasis on conformity assessments and clinical evaluations. Under the MDR, Class IIa and Class IIb devices must adhere to specific common specifications and undergo periodic safety updates, while Class III devices are subjected to rigorous scrutiny throughout their lifecycle.
Grasping these governing frameworks is crucial for manufacturers seeking to ensure compliance and understand the medical devices meaning to enable market access. This is particularly relevant in regions like Latin America, where bioaccess® operates, as navigating these complexities can significantly impact the speed and success of product commercialization.
Colombia offers significant competitive advantages for first-in-human clinical trials, including cost savings of over 30% compared to North America and Western Europe, with regulatory reviews taking only 90-120 days. The country's healthcare system is highly regarded, ranked 22nd by the World Health Organization, and approximately 95% of its population is covered by universal healthcare, facilitating patient recruitment. Additionally, R&D tax incentives provide substantial financial benefits for startups, making Colombia an attractive destination for clinical trials. With the evolving landscape of medical equipment regulations, including anticipated updates in 2025, staying informed is crucial for industry leaders seeking to maintain a competitive edge.

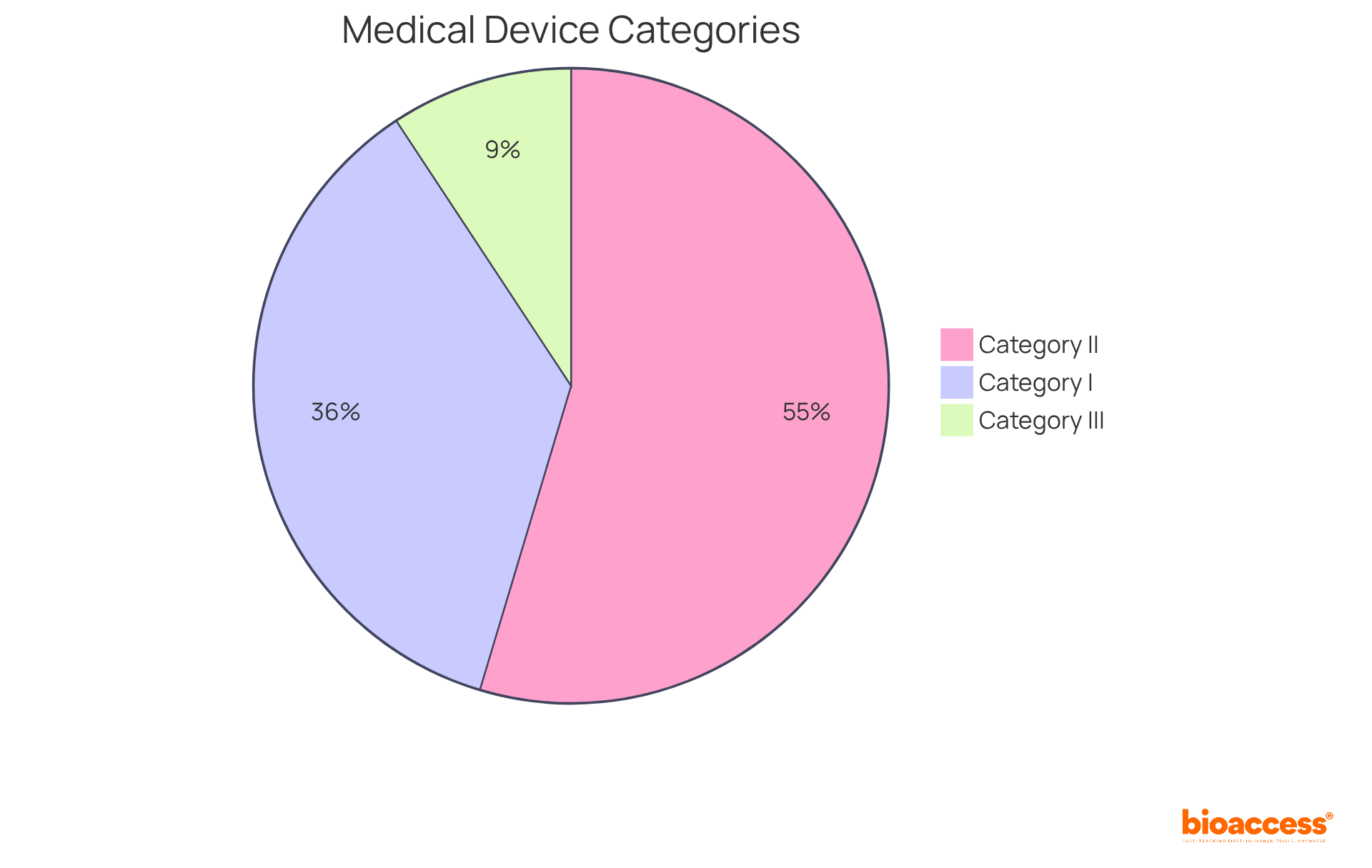
Medical instruments are categorized based on their intended use and associated risks, with the FDA classifying them into three primary categories: I, II, and III.
In Europe, the classification system aligns with similar risk-based criteria, categorizing items into Class I, IIa, IIb, and III under the Medical Devices Regulation (MDR). Understanding these classifications is crucial for producers, as they dictate the medical devices meaning, official pathways, and compliance measures necessary to ensure safety and efficacy. As industry experts highlight, navigating these complexities is vital for successful market entry and adherence to evolving regulations.
The intended purpose of a medical instrument is defined as the specific medical objectives for which it is designed, illustrating the medical devices meaning in terms of the conditions it aims to diagnose, treat, or monitor. This definition is vital, as it directly impacts the classification and regulatory requirements related to medical devices meaning. For instance, equipment aimed at life-sustaining therapies is subject to more rigorous examination compared to those created for general wellness. In fact, a significant percentage of medical equipment is developed specifically for life-sustaining purposes, highlighting the importance of precise classification.
Precise clarification of the intended purpose is crucial for adherence to regulations and ensures that the medical devices meaning effectively addresses the requirements of healthcare providers and patients. Under the EU Medical Device Regulation (MDR), the medical devices meaning must be clearly articulated in technical documentation, as outlined in Article 2(12). This clarity assists manufacturers in evading regulatory pitfalls and guarantees that the product is accurately represented throughout its lifecycle.
Consider a cardiac defibrillator, which is explicitly designed to restore normal heart rhythm in life-threatening situations. Its intended purpose necessitates rigorous clinical evaluation to demonstrate safety and efficacy. Conversely, a fitness tracker, aimed at promoting general wellness, undergoes a different level of scrutiny, reflecting its less critical intended purpose.
The depth and extent of clinical evaluation depend on the intended purpose, classification of the apparatus, and associated risks, all of which are crucial for understanding medical devices meaning. Manufacturers must plan, conduct, and document clinical evaluations to meet general safety and performance requirements, ensuring that the intended purpose is consistently connected to the clinical data pertinent to the product. This structured approach not only facilitates smoother regulatory approval but also enhances patient safety and device effectiveness.

The exploration of medical devices underscores their critical role in enhancing healthcare outcomes and ensuring patient safety. By delving into the multifaceted nature of these instruments—ranging from basic tools to sophisticated technologies—stakeholders can fully appreciate their significance in diagnosing, monitoring, and treating health conditions. The advancements in medical devices, paired with a robust regulatory framework, highlight the necessity of adhering to standards that guarantee safety and efficacy in patient care.
Key arguments presented throughout the article emphasize the transformative impact of medical devices on patient management and surgical precision. Innovations such as robotic-assisted surgeries and wearable health technologies exemplify how these instruments not only enhance patient outcomes but also promote proactive health monitoring. Moreover, the regulatory classifications established by entities like the FDA and the EU's MDR are vital for ensuring that medical devices adhere to the requisite safety and effectiveness standards, thereby safeguarding patients and fostering trust in healthcare systems.
As the landscape of medical devices evolves, it is imperative for manufacturers and healthcare professionals to stay informed about regulatory changes and technological advancements. Embracing these innovations and comprehending their implications can lead to improved health outcomes and more effective patient care. The significance of medical devices in modern medicine cannot be overstated; they are foundational to the future of healthcare, driving enhancements that elevate quality of life and operational efficiency across the sector.
What is a healthcare instrument?
A healthcare instrument encompasses a wide range of items, including tools, equipment, machines, implants, in vitro reagents, and software, all designed for health-related purposes. This includes everything from basic items like bandages to advanced imaging systems.
Why are healthcare instruments important?
Healthcare instruments are crucial because they diagnose, prevent, monitor, and treat various health conditions, making them a fundamental part of modern healthcare.
What advancements have been made in healthcare instruments recently?
Recent advancements, particularly in 2025, have focused on improving accessibility and accuracy. Innovations in robotic systems are enabling minimally invasive procedures that enhance patient outcomes by reducing recovery times and complications.
What is the projected growth of the orthopedic robotics market?
The orthopedic robotics market is expected to exceed $3.5 billion by 2030, with a compound annual growth rate of over 10%.
How do healthcare instruments impact patient care?
Healthcare instruments, such as robotic-assisted surgeries, improve surgical precision, enhance implant longevity, and reduce the need for corrective surgeries. Additionally, wearable health devices enable real-time health monitoring, leading to better management of chronic conditions and fewer hospital visits.
What role does AI and machine learning play in healthcare instruments?
AI and machine learning are increasingly important in health technologies as they enhance diagnostic accuracy and facilitate remote patient monitoring, which can help avoid emergency visits and hospital readmissions, ultimately saving healthcare costs.
What concerns exist regarding the safety and effectiveness of healthcare instruments?
A significant number of healthcare instruments withdrawn due to serious health concerns had gained approval through the less stringent 510(k) process, highlighting the need for better oversight. Furthermore, only 16% of randomly selected implants had publicly accessible scientific evidence supporting their safety and effectiveness.
Why is understanding medical devices significant for stakeholders in the health sector?
Understanding the meaning and significance of medical devices is crucial for stakeholders as it informs compliance with regulations, product development, and market access strategies, which ultimately leads to improved patient care and health outcomes.