


The article examines the definition, evolution, and impact of pharma medicine, highlighting its essential role in the development and regulation of new medications that improve patient care and public health. It details the rigorous processes involved in drug development, outlines the historical advancements that have shaped the field, and addresses current trends, including the integration of advanced technologies and the necessity for diverse clinical trial participation. These elements collectively influence treatment efficacy and accessibility, underscoring the importance of collaboration in advancing clinical research.
Pharma medicine stands at the intersection of scientific innovation and patient care, shaping the landscape of modern healthcare through the development of new medications. This specialized field evolves continuously, offering significant opportunities for improving treatment efficacy and patient outcomes. However, it also faces challenges related to drug pricing and trial diversity.
How can the pharmaceutical industry balance the rapid advancement of medical technologies with the pressing need for equitable access to these innovations?
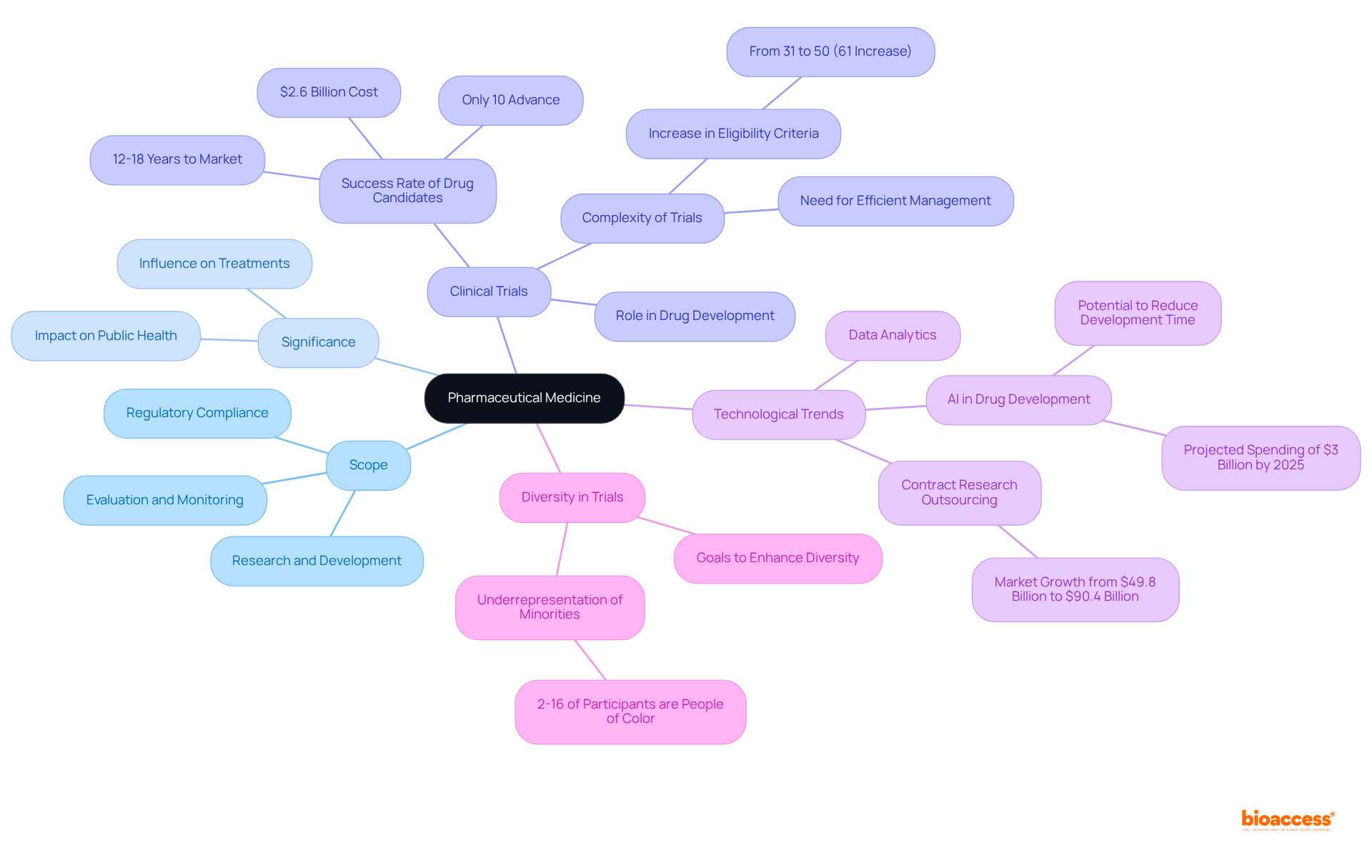
Pharma medicine, often referred to as pharmaceutical medicine, represents a specialized domain within the medical field that concentrates on the research, development, evaluation, registration, and monitoring of new medications. This field encompasses a wide range of activities, such as clinical studies, regulatory compliance, and post-marketing surveillance. The significance of pharma medicine is profound, as it directly influences the availability and efficacy of treatments for various diseases, ultimately shaping patient outcomes and public health. This discipline serves as a vital bridge between scientific research and clinical application, ensuring that new therapies are safe, effective, and accessible to patients.
The critical role of clinical trials within this framework cannot be overstated. They form the backbone of pharmaceutical advancement, providing essential data that informs regulatory decisions and guides treatment protocols. Statistics reveal that only 10% of drug candidates successfully advance to clinical development, with the journey to market taking 12 to 18 years and approximately $2.6 billion. This highlights the rigorous nature of the process. Furthermore, the complexity of clinical studies has increased significantly, with the average number of eligibility criteria rising from 31 to 50 between 2001 and 2015, marking a 61% increase. This complexity underscores the necessity for efficient management and innovative strategies in study design to enhance patient recruitment and retention.
Current trends in medical science indicate a growing reliance on advanced technologies, such as artificial intelligence and data analysis, to streamline medication development and enhance research efficiency. Amgen has observed that AI could potentially 'shave two years off the decade or more it typically takes to develop a drug.' The global market for contract research outsourcing, which underpins these initiatives, is anticipated to expand from $49.8 billion in 2022 to $90.4 billion by 2030, fueled by the rising demand for expertise and operational flexibility. As pharmaceutical companies navigate these evolving landscapes, prioritizing the inclusion of diverse patient demographics in clinical studies has become essential. Despite individuals of color representing approximately 39% of the US population, they constitute only 2% to 16% of study participants. More than half of surveyed companies have set goals to enhance trial diversity, striving to improve the representativeness of trial participants and ensure that new treatments cater to the needs of all patient demographics, ultimately leading to improved health outcomes.
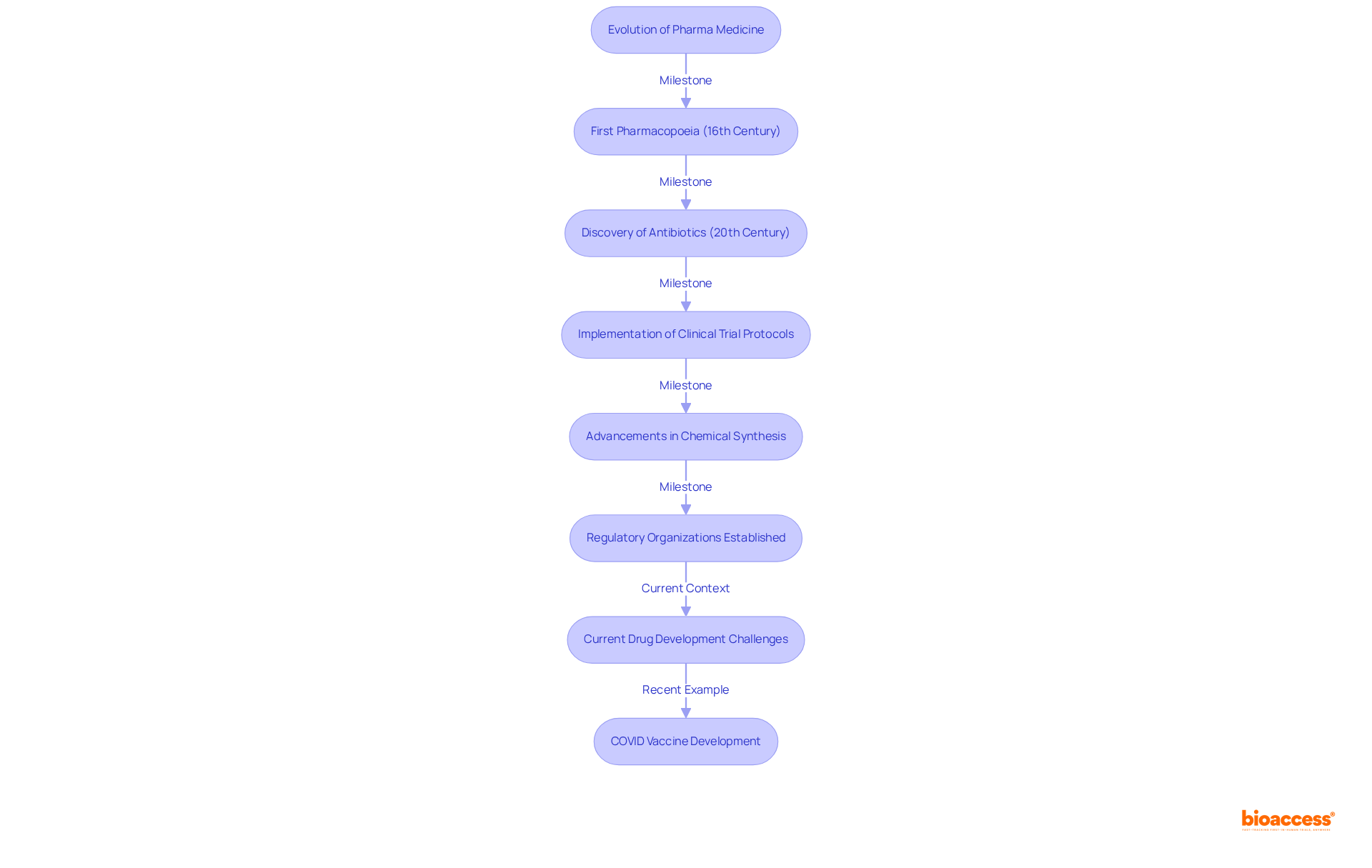
The development of medicinal practices traces back to ancient methods, where herbal treatments served as the primary means of addressing ailments. The contemporary pharma medicine sector began to take shape in the 19th century, driven by advancements in chemical synthesis and the establishment of regulatory organizations that ensured the safety and effectiveness of medications. Notable milestones in this evolution include:
Over the years, technological advancements and a deeper understanding of disease mechanisms have enhanced medication development processes. Currently, the typical timeframe to bring a new pharma medicine to market exceeds 10 years, with costs ranging from $985 million to $2.6 billion, underscoring the challenges in ensuring the safety and efficacy of pharma medicine. Significantly, the average probability of approval for medications in Phase I is merely 7.9%, illustrating the hurdles faced in drug development. Regulatory bodies, such as the U.S. Food and Drug Administration (FDA), have been instrumental in shaping these processes, introducing initiatives to expedite approvals and bolster public trust in pharma medicine. The rapid development of COVID vaccines exemplified that with sufficient funding and political will, medical development timelines can be dramatically shortened, serving as a contemporary case study of innovation in the sector. As the industry continues to advance, the focus on evidence-based medicine and regulatory compliance remains critical, ensuring that innovations translate into safe and effective treatments for patients.
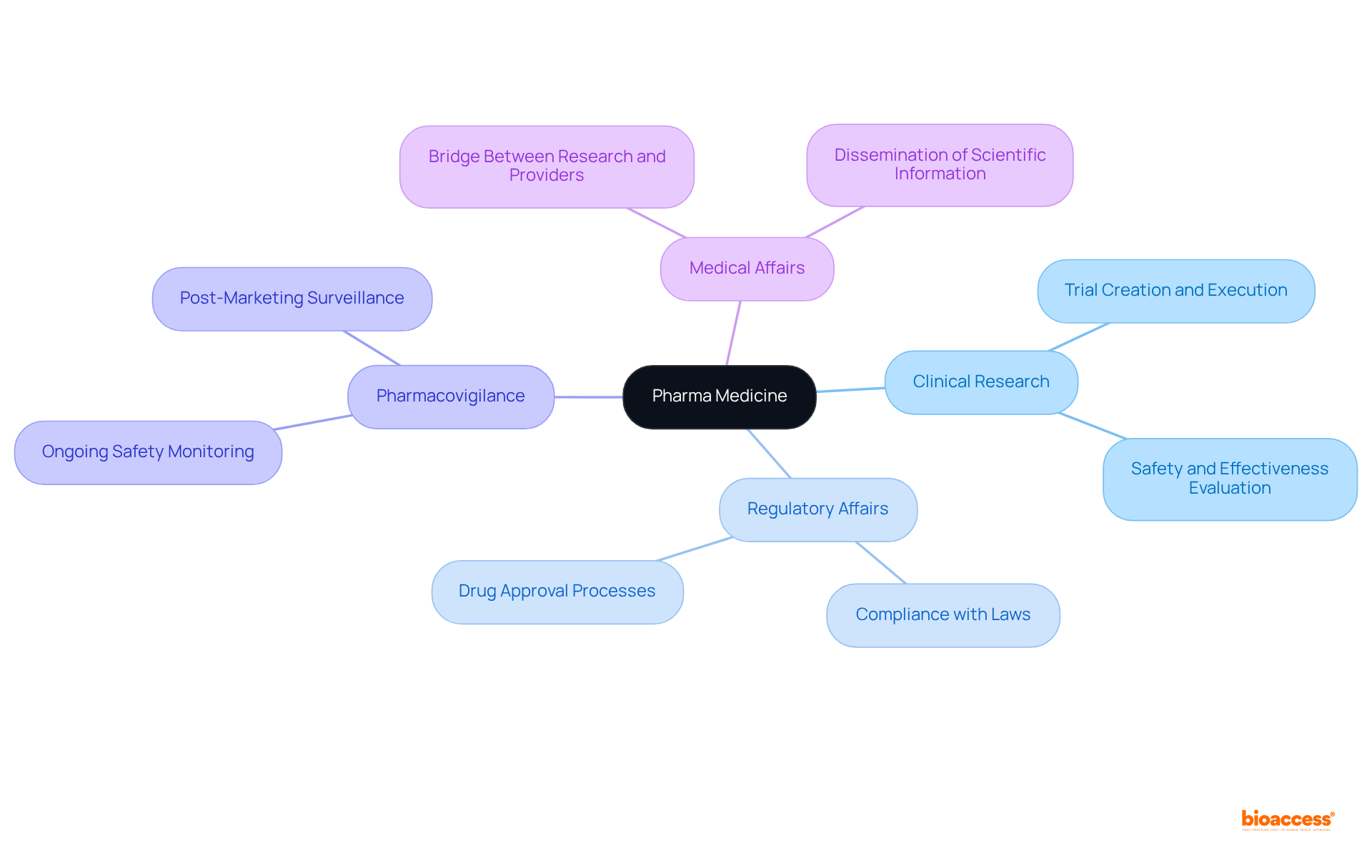
Several key components characterize pharma medicine, including:
Clinical research involves the creation and execution of trials aimed at evaluating the safety and effectiveness of new medications. Regulatory affairs play a critical role in ensuring compliance with local and international laws governing drug approval and marketing. Pharmacovigilance focuses on the ongoing monitoring of medication safety post-approval. Meanwhile, medical affairs serve as a bridge between clinical research and healthcare providers by disseminating essential scientific information. Together, these elements form a robust structure that facilitates the creation and provision of safe and effective pharma medicine.
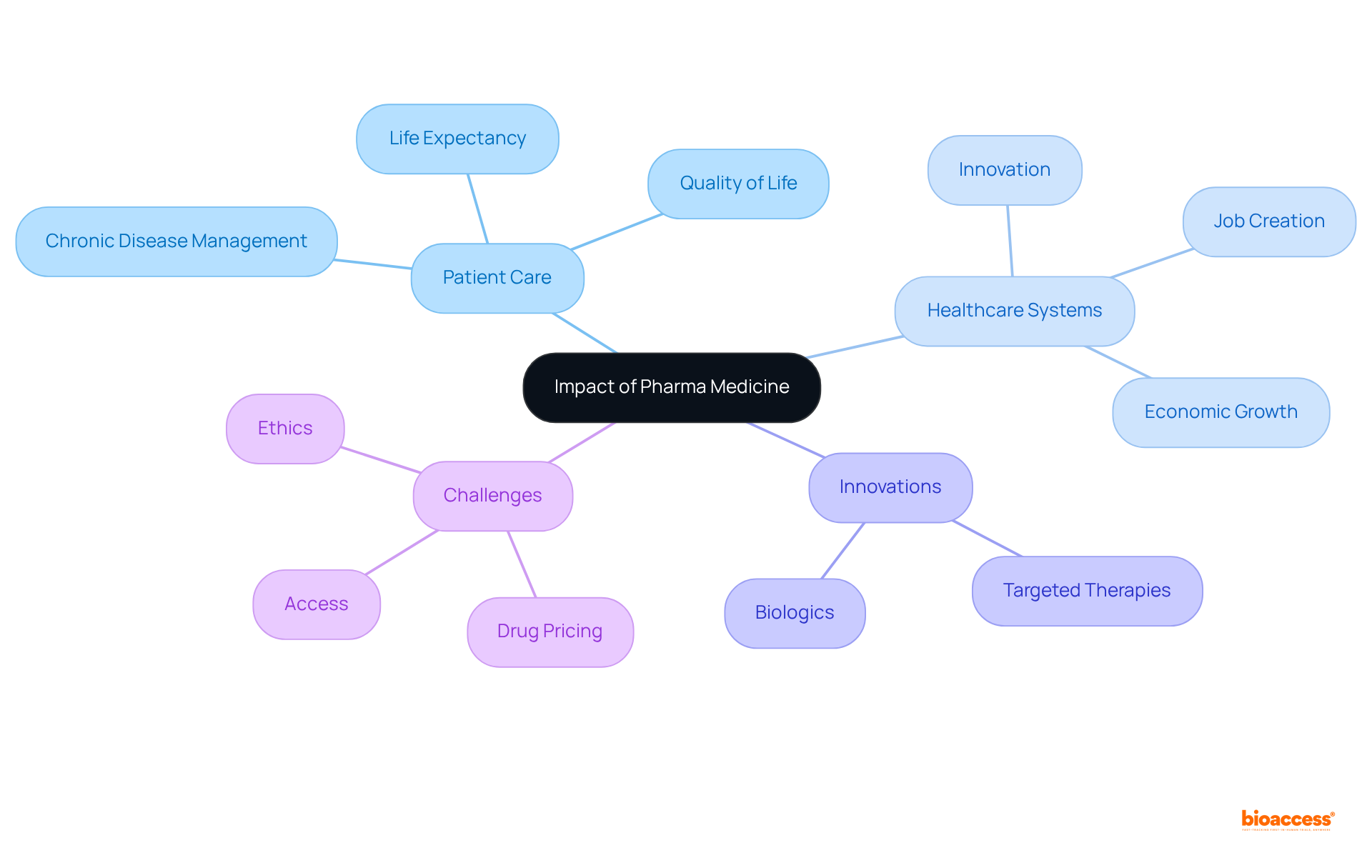
Pharma medicine significantly contributes to enhancing patient care and improving healthcare systems. The development of new medications has led to better management of chronic diseases, improved quality of life, and increased life expectancy. For instance, the introduction of targeted therapies and biologics has revolutionized the treatment of conditions such as cancer and autoimmune diseases.
Moreover, statistics show that medical innovation has led to a significant rise in life expectancy, with estimates suggesting that 66% of the increase in average age at death in the U.S. from 2006 to 2018 can be linked to progress in medicine. The typical quantity of new medications authorized annually from 2010 to 2019 was 60% greater than the prior decade, indicating substantial advancement in medical innovation.
Additionally, the pharmaceutical industry contributes to healthcare systems by driving innovation, creating jobs, and supporting economic growth. However, challenges such as drug pricing, access to medications, and ethical considerations in clinical trials remain critical issues that need to be addressed to maximize the benefits of pharma medicine.
Insights from healthcare professionals underscore the importance of these new medications in chronic disease management, highlighting their role in improving patient outcomes and overall healthcare effectiveness.
Pharma medicine plays a crucial role in the landscape of modern healthcare, encompassing the research, development, and regulation of new medications that directly impact patient outcomes. This specialized field not only bridges scientific advancement and clinical application but also ensures that treatments are safe, effective, and accessible. The evolution of pharma medicine, from its historical roots to the present-day complexities, illustrates its significance in improving health outcomes and shaping public health policies.
Key insights from the article highlight the rigorous processes involved in drug development, including:
These elements work in tandem to enhance the efficiency of medication development while addressing critical issues such as trial diversity and patient representation. Furthermore, the evolution of pharma medicine has led to remarkable advancements in treating chronic conditions, significantly increasing life expectancy and improving the quality of life for countless individuals.
As the pharmaceutical industry continues to evolve, it is essential to recognize the challenges that accompany these advancements, such as:
A commitment to addressing these issues will maximize the benefits of pharma medicine, ensuring that innovations translate into meaningful improvements in patient care and healthcare systems. Embracing the complexities of this field will empower stakeholders to foster a more inclusive and effective healthcare environment, ultimately leading to better health outcomes for all.
What is pharma medicine?
Pharma medicine, also known as pharmaceutical medicine, is a specialized field that focuses on the research, development, evaluation, registration, and monitoring of new medications. It includes activities such as clinical studies, regulatory compliance, and post-marketing surveillance.
Why is pharma medicine significant?
Pharma medicine is significant because it directly influences the availability and efficacy of treatments for various diseases, shaping patient outcomes and public health. It acts as a crucial link between scientific research and clinical application, ensuring that new therapies are safe, effective, and accessible to patients.
What role do clinical trials play in pharma medicine?
Clinical trials are essential in pharma medicine as they provide critical data that informs regulatory decisions and guides treatment protocols. They form the backbone of pharmaceutical advancement.
How successful are drug candidates in clinical development?
Statistics indicate that only 10% of drug candidates successfully advance to clinical development, and the journey to market typically takes 12 to 18 years and costs approximately $2.6 billion.
What trends are influencing clinical studies?
The complexity of clinical studies has increased, with the average number of eligibility criteria rising from 31 to 50 between 2001 and 2015. This complexity necessitates efficient management and innovative strategies in study design to improve patient recruitment and retention.
How is technology impacting medication development?
Advanced technologies, such as artificial intelligence and data analysis, are increasingly being used to streamline medication development and enhance research efficiency. For example, Amgen has noted that AI could potentially reduce the time it takes to develop a drug by two years.
What is the projected growth of the contract research outsourcing market?
The global market for contract research outsourcing is expected to grow from $49.8 billion in 2022 to $90.4 billion by 2030, driven by the demand for expertise and operational flexibility in the pharmaceutical industry.
What is being done to improve diversity in clinical studies?
There is a growing emphasis on including diverse patient demographics in clinical studies. Despite individuals of color making up about 39% of the US population, they represent only 2% to 16% of study participants. More than half of surveyed companies have set goals to enhance trial diversity to ensure new treatments address the needs of all patient demographics and improve health outcomes.