


Phase Zero clinical trials play a pivotal role in providing early insights into the pharmacokinetics and pharmacodynamics of new compounds through the administration of microdoses to a select group of participants. These trials are essential for identifying promising drug candidates while effectively minimizing risks and costs. By allowing researchers to gather critical data prior to advancing to more extensive phases of clinical development, Phase Zero trials set the foundation for informed decision-making in the drug development process.
Phase Zero clinical trials represent a pivotal advancement in pharmaceutical innovation, offering a distinctive insight into the initial phases of drug development. These exploratory studies, which involve administering microdoses to a limited number of volunteers, yield essential information regarding a drug's interaction within the human body, thereby setting the stage for more comprehensive research.
As the clinical trial landscape continues to evolve, however, challenges related to patient recruitment and ethical considerations arise, prompting critical inquiries about the equilibrium between scientific progress and participant welfare.
What implications arise from conducting a trial devoid of therapeutic intent, and how can researchers adeptly navigate the intricacies of ethical compliance while pursuing significant outcomes?
Phase Zero clinical trials, which are also referred to as exploratory investigational new substance (IND) studies, represent the foundational stage of clinical research. During this phase, a proposed compound is administered to a limited cohort of human volunteers, typically numbering fewer than 15. These studies employ subtherapeutic doses, commonly referred to as microdoses, to collect preliminary data regarding the substance's pharmacokinetics (PK) and pharmacodynamics (PD), without the intention of providing therapeutic benefits. The primary aim is to assess the drug's behavior within the human body, encompassing its absorption, distribution, metabolism, and excretion—critical information that informs subsequent phases of clinical development.
However, the rapid recruitment of patients for these early-phase clinical studies poses significant challenges. Potential participants often exhibit hesitance, face numerous options, or may be disqualified. This issue particularly impacts Medtech, Biopharma, and Radiopharma startups, which frequently lack the financial resources and infrastructure necessary to efficiently conduct their studies, recruit patients, and gather data for analysis. bioaccess offers innovative solutions designed to streamline patient recruitment processes, thereby enhancing the efficiency of trial management.
Recent advancements in exploratory IND studies underscore their pivotal role in the phase zero clinical trial of medication development. For instance, the FDA's guidance on exploratory INDs has enabled the implementation of microdosing techniques, allowing researchers to acquire valuable insights into drug behavior at very low doses, sometimes as minimal as 100 nanocuries. This methodology not only mitigates the risk of adverse effects but also accelerates the clinical development process by facilitating the early identification of ineffective agents.
Statistical designs for preliminary studies are meticulously tailored to uphold rigor, even with limited sample sizes. A one-stage design, for example, can yield approximately 90% power to detect a true pharmacodynamic response rate of 80% among patients. This statistical framework is essential for assessing treatment-related changes from baseline in pharmacodynamic endpoints, ideally measured in both tumor and surrogate tissues. Additionally, two-stage designs may be employed to further enhance the statistical reliability of these studies.
Ethical considerations associated with initial studies are paramount, given that these investigations lack therapeutic intent. Informed consent procedures must guarantee that participants fully comprehend the study's nature and the absence of therapeutic benefits. This transparency is vital for fostering trust and compliance among volunteers. Furthermore, the lack of therapeutic purpose can deter volunteer participation, as potential participants might be discouraged by the absence of direct benefits.
In summary, initial testing procedures serve as a crucial instrument in the medication development landscape, providing early proof-of-concept evaluations and refining target or biomarker assay techniques before larger studies commence. Their significance is anticipated to grow as methodologies evolve and data sharing improves, ultimately enhancing the efficiency and success of clinical research. Notable examples of exploratory IND studies, such as the NCI study of ABT-888 and the SR13668 study, illustrate the practical implications of these investigations in advancing medication development.

The primary objective of Stage One studies is to provide initial insights into the pharmacokinetic (PK) and pharmacodynamic (PD) characteristics of a candidate compound. By employing microdoses—defined as less than 1/100th of the pharmacologically active dose—researchers can gather crucial data that assists in evaluating the potential effectiveness of a substance in larger populations. This phase is particularly significant as it facilitates the identification of promising candidates while minimizing risks to participants; exposure to subtherapeutic doses diminishes the likelihood of adverse effects.
Moreover, initial experiments lead to substantial cost reductions in the medication development process. By eliminating ineffective compounds before they progress to more comprehensive Stage I evaluations, these studies can decrease the total time and financial resources required for medication development. For instance, initial programs typically incur expenses between $0.7 million and $1.25 million, which is approximately one-third of the cost of conventional first-phase studies, which can range from $1.75 million to $3.5 million. This preliminary assessment not only optimizes resource allocation but also enhances the probability of success in subsequent stages, ultimately resulting in a more effective treatment development pipeline.
Real-world examples underscore the effectiveness of initial testing in reducing medication development costs. The National Cancer Institute's phase zero clinical trial of ABT-888, for example, demonstrated a statistically significant reduction in PAR levels, providing critical insights that informed further development decisions. Such experiments exemplify how early pharmacokinetic and pharmacodynamic information can be utilized in a phase zero clinical trial to guide the selection of viable therapeutic candidates, thereby improving the overall development process.
Expert opinions emphasize the importance of these evaluations in the context of medication advancement. By enabling earlier human testing with reduced preclinical toxicology requirements, preliminary studies foster a more adaptable approach to assessing new molecular entities. This strategic advantage not only accelerates the validation of targets and biomarkers but also enhances the overall efficiency of drug development, ultimately benefiting both researchers and patients alike.
Furthermore, the FDA's exploratory IND guidance issued in 2006 supports clinical assessment prior to traditional IND studies, further illustrating the regulatory framework that facilitates initial studies. The regulatory review period for these studies is notably shorter, at 30 days in the US, compared to the extended timelines for conventional first-stage studies. Additionally, the initial stage of testing generally involves fewer participants, typically ranging from 4 to 10, as opposed to the 6 to 30 participants in standard first-stage studies, highlighting their effectiveness and lower risk. Ethical considerations surrounding the initial stages of experiments, particularly the absence of therapeutic intent and the importance of informed consent, are crucial for understanding the context of these studies. Overall, the initial testing stages play a vital role in prioritizing investigational agents for future studies, ultimately enhancing the medication development process.
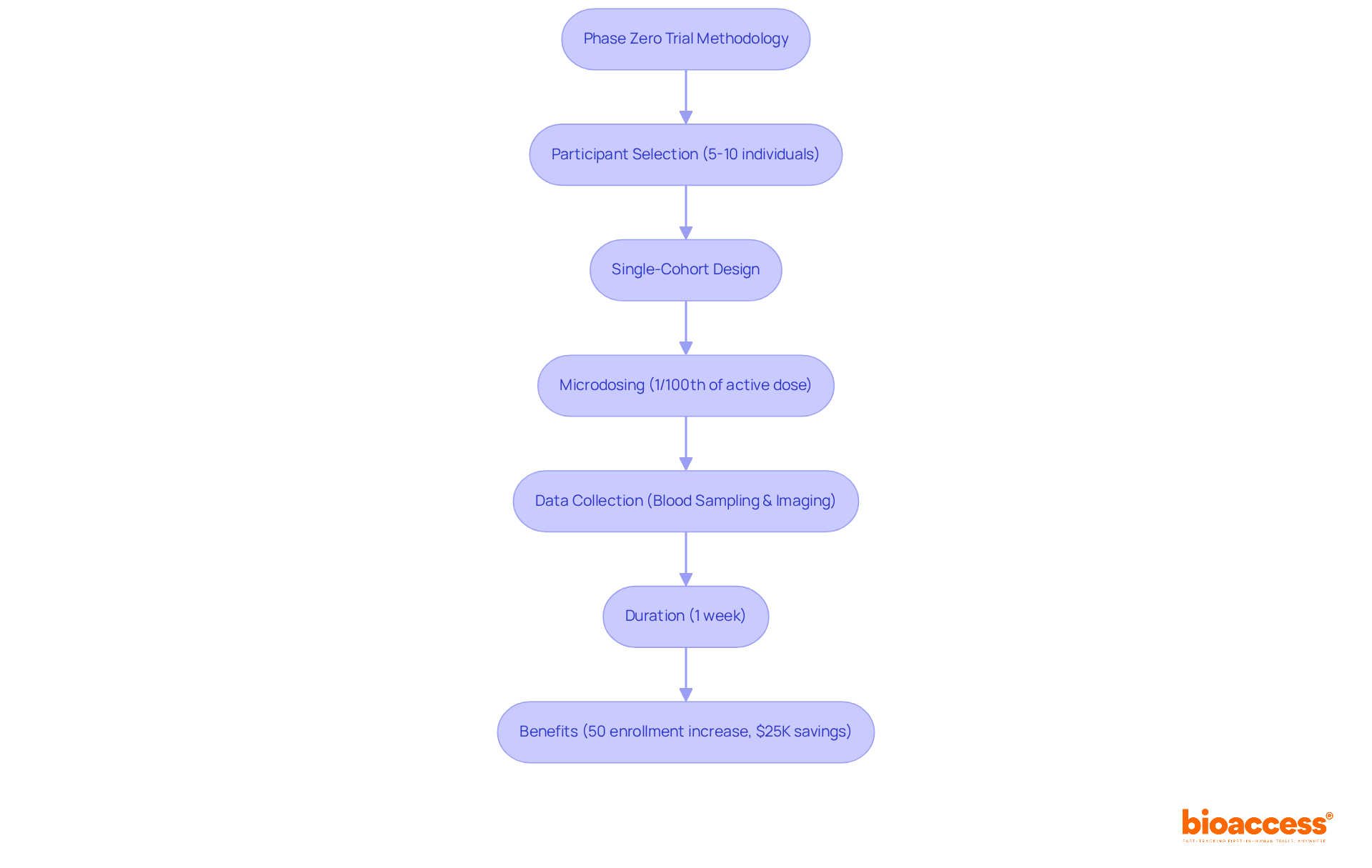
Initial studies are characterized by their small participant groups, typically comprising 5 to 10 individuals selected based on stringent inclusion and exclusion criteria tailored for the experimental medication. These studies frequently utilize a single-cohort design, wherein all participants receive the same microdose—defined as 1/100th of the pharmacologically active dose or the no observed adverse effect level (NOAEL). This meticulous dosing strategy not only minimizes risk but also facilitates the collection of essential pharmacokinetic and pharmacodynamic data. Data collection methods often incorporate blood sampling to assess substance concentration alongside advanced imaging techniques to illustrate effects on target tissues.
The duration of a phase zero clinical trial is generally brief, often limited to one week, which allows for rapid data collection and analysis. This accelerated timeline is vital for informing the phase zero clinical trial and optimizing resource allocation in drug development. With Bioaccess's comprehensive clinical study management services—including feasibility studies, study setup, compliance reviews, site selection, and project management—these investigations can be executed efficiently. Notably, Bioaccess enhances patient enrollment by 50% and delivers FDA-ready data, ensuring that the studies are not only swift but also cost-effective, saving approximately $25K for each patient.
Ethical considerations in Phase Zero research are paramount, as these studies do not aim to provide therapeutic advantages to participants. Informed consent is critical; participants must be fully aware that they are receiving a microdose of a drug without any guaranteed health benefits. Statistics reveal a concerning variance in understanding the components of informed consent, with only 54.9% of participants able to identify at least one risk associated with their involvement, while comprehension of the study's purpose is at 69.6% and the voluntary nature of participation at 74.7%.
Regulatory bodies, including the FDA and EMA, have established guidelines to ensure ethical conduct in these studies, adhering to principles outlined in the Declaration of Helsinki. Notably, the FDA's Exploratory IND Guidance, released in 2006, supports the implementation of Phase Zero studies, emphasizing the necessity for strict compliance with established protocols.
Researchers must diligently evaluate potential risks and benefits, designing studies that minimize harm while maximizing valuable data collection. Continuous observation and transparent communication with participants are essential to uphold ethical standards throughout the research process.
Furthermore, expert insights underscore the importance of regulatory compliance in Phase Zero studies, particularly regarding exploratory IND research, which requires stringent adherence to established protocols to ensure participant safety and data integrity. Ethical concerns also emerge regarding the recruitment of individuals who may derive no potential clinical benefit, especially terminally ill patients, highlighting the complexities inherent in these trials.

Phase Zero clinical trials serve as a critical entry point in the medication development process, focusing on the initial exploration of a drug's pharmacokinetics and pharmacodynamics through the administration of microdoses. These trials are designed to gather essential data without therapeutic intent, allowing researchers to evaluate the safety and behavior of new compounds before advancing to larger clinical studies. The significance of these trials lies in their ability to streamline the drug development pipeline, reduce costs, and enhance the likelihood of identifying promising therapeutic candidates.
The article highlights several key aspects of Phase Zero trials, including their purpose, methodology, and ethical considerations. By utilizing small participant groups and rigorous statistical designs, these studies can yield valuable insights while minimizing risks associated with drug exposure. Furthermore, the ethical imperative of informed consent is emphasized, ensuring that participants are fully aware of the nature of the trial and the absence of direct therapeutic benefits. Regulatory frameworks, such as the FDA's Exploratory IND Guidance, play a vital role in maintaining the integrity and safety of these studies.
Ultimately, Phase Zero clinical trials represent an innovative approach to drug development that not only accelerates the research process but also prioritizes participant safety and informed decision-making. As methodologies evolve and the landscape of clinical research continues to transform, the importance of these early-stage studies will likely grow, paving the way for more effective and efficient medication development. Embracing the potential of Phase Zero trials can lead to significant advancements in healthcare, benefiting both researchers and patients alike.
What are Phase Zero clinical trials?
Phase Zero clinical trials, also known as exploratory investigational new substance (IND) studies, represent the foundational stage of clinical research where a proposed compound is administered to a small group of human volunteers, typically fewer than 15, using subtherapeutic doses to collect preliminary data on the drug's pharmacokinetics and pharmacodynamics.
What is the primary aim of Phase Zero clinical trials?
The primary aim is to assess the drug's behavior within the human body, including its absorption, distribution, metabolism, and excretion, to gather critical information for subsequent phases of clinical development.
What challenges do researchers face in recruiting participants for Phase Zero trials?
Researchers often encounter challenges such as participant hesitance, numerous options available to potential participants, and disqualifications. This issue is particularly pronounced for Medtech, Biopharma, and Radiopharma startups that may lack the resources and infrastructure for efficient patient recruitment.
How does bioaccess contribute to Phase Zero clinical trials?
bioaccess offers innovative solutions designed to streamline patient recruitment processes, thereby enhancing the efficiency of trial management in early-phase clinical studies.
What advancements have been made in exploratory IND studies?
Recent advancements include the FDA's guidance on exploratory INDs, which has enabled the use of microdosing techniques. This allows researchers to gain insights into drug behavior at very low doses, reducing the risk of adverse effects and accelerating the clinical development process.
What statistical designs are used in Phase Zero clinical trials?
Statistical designs for preliminary studies are tailored for rigor, with one-stage designs providing approximately 90% power to detect a true pharmacodynamic response rate of 80%. Two-stage designs may also be used to enhance statistical reliability.
What ethical considerations are important in Phase Zero trials?
Ethical considerations include ensuring informed consent, where participants must fully understand the study's nature and the absence of therapeutic benefits. Transparency is vital for fostering trust and compliance, though the lack of therapeutic purpose may deter potential volunteers.
What is the significance of initial testing procedures in medication development?
Initial testing procedures provide early proof-of-concept evaluations and refine target or biomarker assay techniques before larger studies. Their significance is expected to grow as methodologies evolve and data sharing improves, enhancing the efficiency and success of clinical research.
Can you provide examples of exploratory IND studies?
Notable examples include the NCI study of ABT-888 and the SR13668 study, which illustrate the practical implications of exploratory IND investigations in advancing medication development.