


Pharmacokinetics (PK) is a critical field that examines how drugs are absorbed, distributed, metabolized, and excreted (ADME) by the body. This understanding is essential for determining appropriate dosages and ensuring drug safety and efficacy. By grasping these processes, healthcare professionals can optimize medication therapy, which in turn enhances drug development and regulatory compliance. This highlights the evolution and significance of PK within clinical pharmacology, reinforcing its vital role in advancing healthcare outcomes.
Pharmacokinetics, a pivotal branch of pharmacology, examines the intricate interplay between the body and drugs, influencing how medications are absorbed, distributed, metabolized, and excreted. This dynamic field not only informs optimal dosing and enhances therapeutic efficacy but also plays a crucial role in patient safety and regulatory compliance.
As drug interactions and individual responses complicate treatment plans, healthcare professionals must leverage pharmacokinetics to navigate these challenges effectively and improve patient outcomes. This exploration is essential for advancing clinical research and ensuring the highest standards of care.
PK pharmacokinetics represents a crucial branch of pharmacology, dedicated to the study of how the body interacts with a drug over time. This field encompasses the essential processes of absorption, distribution, metabolism, and excretion (ADME). Understanding drug metabolism is vital; it allows for the determination of appropriate medication dosages, predicts their effects, and ensures safety and efficacy.
By examining the pathways medications traverse within the body, researchers can enhance treatment plans and mitigate adverse effects. This establishes PK pharmacokinetics as a fundamental aspect of clinical pharmacology, underscoring its importance in advancing healthcare outcomes.

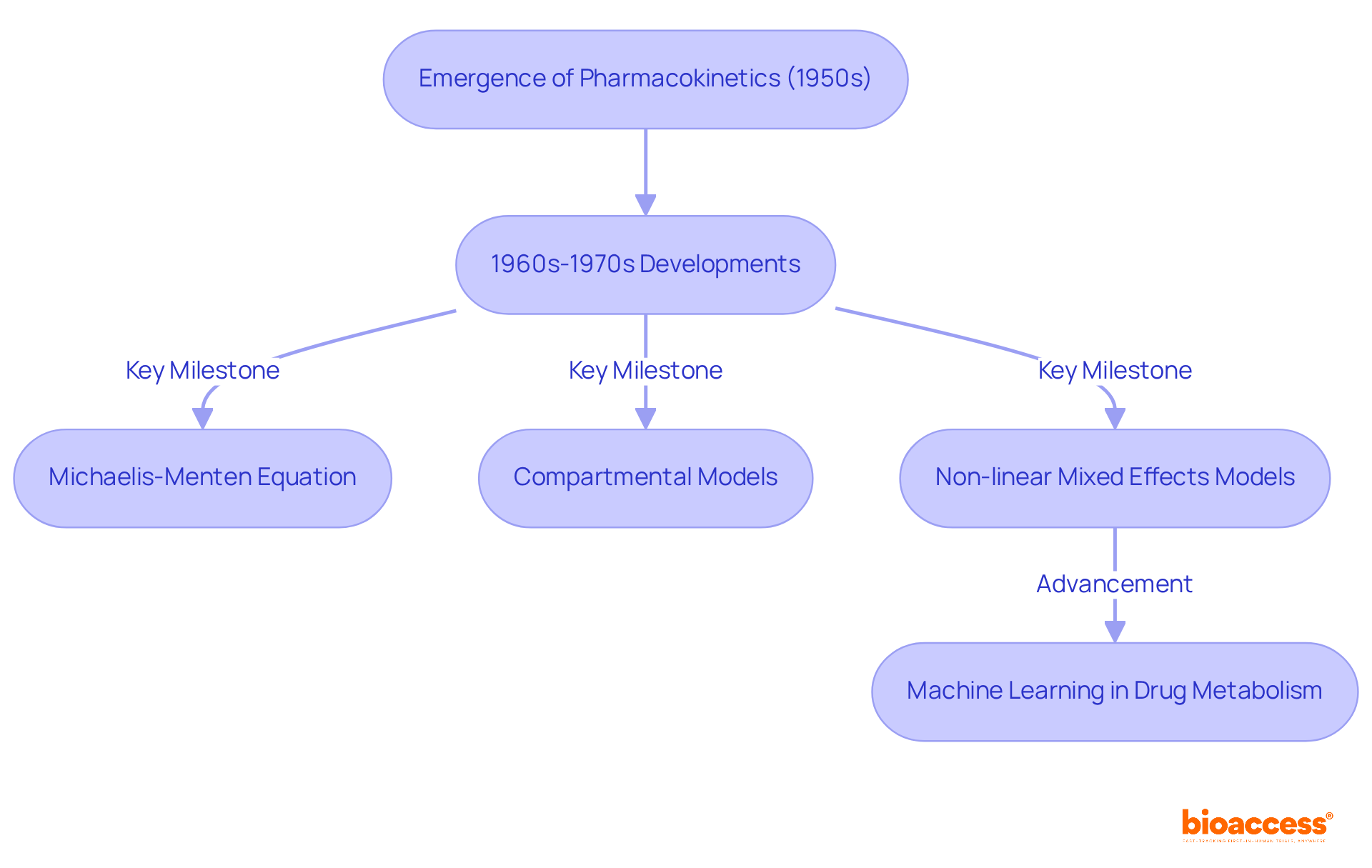
The term 'pharmacokinetics' emerged in the early 1950s, marked by pivotal contributions from researchers such as Friedrich Hartmut Dost, who laid the groundwork for this essential field. The 1960s and 1970s signified a notable acceleration in the study of substances within the body, driven by advancements in mathematical modeling and an increasing demand for precise evaluation techniques. Significant breakthroughs during this period included:
As pharmacokinetics evolved, it became integral to medication development and regulatory practices, ensuring that therapies are both effective and safe for individuals. The progression of this discipline has been characterized by the application of sophisticated mathematical models, which enhance the ability to predict drug behavior and pharmacokinetics across various populations. For instance, the introduction of non-linear mixed effects models represented a significant shift in pharmacokinetics analysis, facilitating a better evaluation of variability in pharmacokinetic parameters among diverse patient groups.
Case studies illustrate the impact of these advancements; for example, the utilization of machine learning methods in drug metabolism has revolutionized data analysis, enabling researchers to integrate larger datasets and improve predictive accuracy. This ongoing evolution underscores the importance of pharmacokinetics within the broader context of medication development, where comprehensive analysis and modeling are vital for informed decision-making and regulatory compliance.
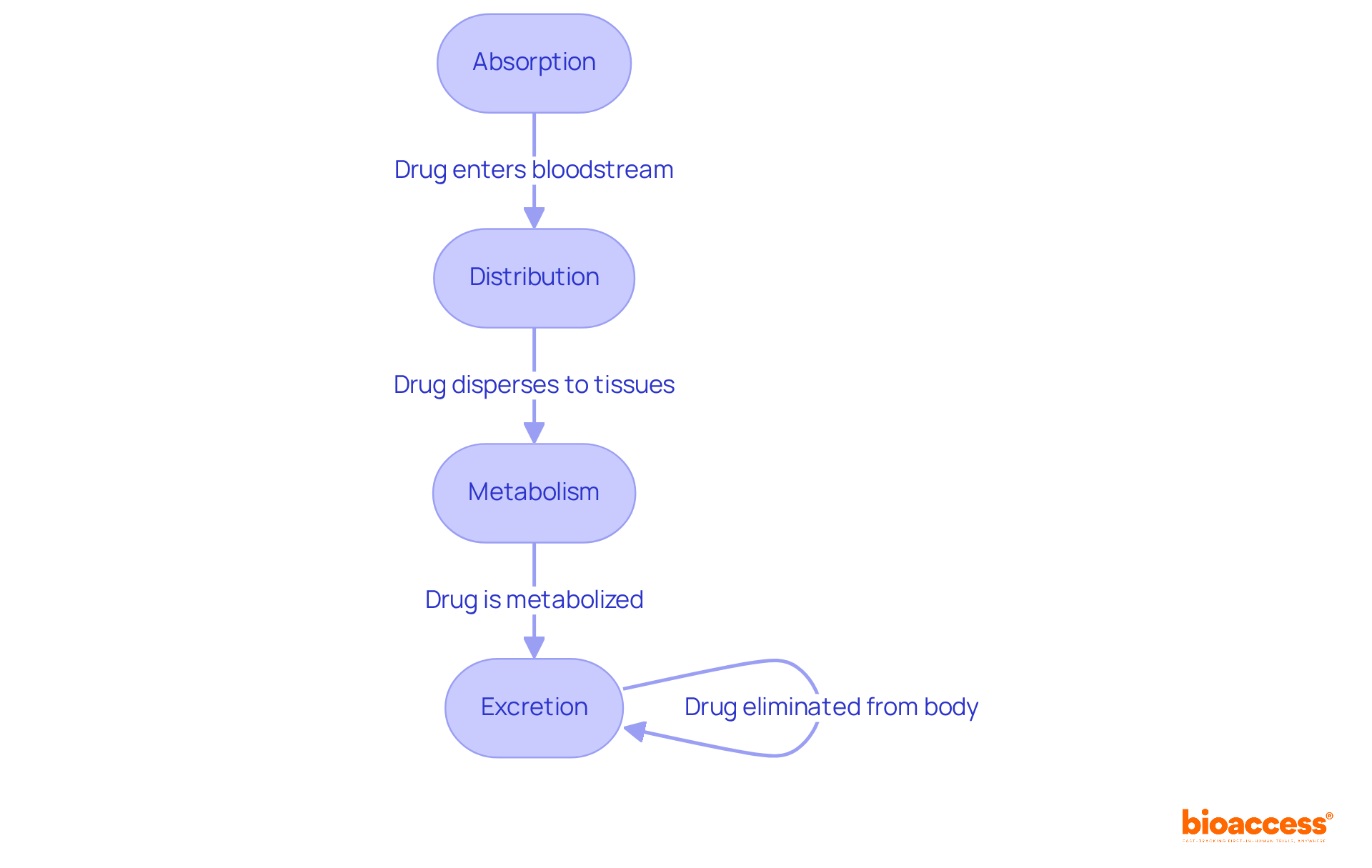
Pk pharmacokinetics centers on four pivotal activities: Absorption, Distribution, Metabolism, and Excretion (ADME), each integral to medication therapy.
Absorption describes how a medication enters the bloodstream from its site of administration. Factors such as the medication's formulation and the route of administration—be it oral, intravenous, or otherwise—significantly influence absorption rates. Notably, intravenous (IV) substances achieve 100% bioavailability, whereas oral medications often face challenges due to first-pass metabolism, which can reduce their effectiveness. The hierarchy of bioavailability among various routes of administration ranks as parenteral, rectal, oral, and topical, respectively.
Distribution pertains to the dispersion of the substance throughout the body, reaching diverse tissues. This process is influenced by blood circulation, tissue permeability, and the substance's affinity for different tissues. For example, lipid-soluble substances can seamlessly traverse biological membranes, allowing them to infiltrate lipid-rich tissues such as the brain and liver, while water-soluble compounds typically remain in aqueous environments.
Metabolism involves the biochemical transformation of the substance, primarily occurring in the liver. This process can either activate or deactivate the medication, significantly impacting its therapeutic effects and potential toxicity. Understanding the cytochrome P-450 system is crucial, as it plays a vital role in substance metabolism, with genetic variations affecting individual responses to treatments. Additionally, dietary factors can influence medication bioavailability, necessitating careful monitoring of patients' diets, particularly for substances like warfarin.
Excretion represents the concluding phase, wherein the substance and its metabolites are eliminated from the body, primarily via urine or feces. Regular monitoring of laboratory values is essential to ensure that medications remain within therapeutic ranges, preventing accumulation and toxicity. A study highlighted that 61% of individuals have one or more prescriptions that are undocumented, increasing the risk of adverse medication events. Furthermore, the role of nurses in medication administration is critical; their understanding of bioavailability can enhance safety by ensuring accurate dosing and vigilant monitoring.
Grasping these ADME processes is vital for optimizing medication therapy and ensuring patient safety, particularly in the realm of pk pharmacokinetics. By thoroughly assessing absorption, distribution, metabolism, and excretion, healthcare professionals can make informed decisions that enhance medication effectiveness and mitigate risks.
PK pharmacokinetics stands as a cornerstone in clinical research, particularly within the realm of medication development. By scrutinizing the pk pharmacokinetics processes of absorption, distribution, metabolism, and excretion of medications in the body, researchers can design clinical trials that not only enhance therapeutic outcomes but also mitigate adverse effects. For instance, pk pharmacokinetics studies are pivotal in determining optimal dosing regimens that yield the desired therapeutic effects. A notable example is the advancement of erlotinib for non-small cell lung cancer, where high-dose pulsed regimens have been shown to effectively combat resistance and improve treatment efficacy, particularly in patients with CNS metastases.
Moreover, a comprehensive understanding of pk pharmacokinetics is vital for assessing medication interactions, which can significantly influence individual patient outcomes. The widespread occurrence of polypharmacy, especially among oncology patients, raises critical concerns regarding potential drug-drug interactions that could undermine treatment efficacy. Regulatory bodies, including the FDA, mandate pk pharmacokinetics data to evaluate new medications, ensuring they satisfy safety and efficacy standards before reaching the market.
The impact of drug metabolism transcends mere regulatory compliance; it enriches the medication development process by offering essential insights that guide clinical decision-making. For example, the MABEL approach is advocated for monoclonal antibodies to determine first-in-human doses based on biological effects, thereby facilitating a safer and more effective transition from preclinical to clinical phases. In summary, pk pharmacokinetics serves as an indispensable foundation in the formulation of effective and safe therapeutic strategies, steering researchers in their pursuit to enhance patient care.

Understanding pharmacokinetics is essential for grasping how medications interact with the body over time, encompassing the critical processes of absorption, distribution, metabolism, and excretion (ADME). This field not only informs the safe and effective use of drugs but also plays a pivotal role in developing treatment protocols that enhance patient outcomes.
The historical evolution of pharmacokinetics is noteworthy, tracing its roots back to the 1950s and showcasing significant advancements that have shaped the discipline. Key components, such as the formulation of the Michaelis-Menten equation and the introduction of compartmental models, illustrate the sophistication of pharmacokinetic analysis. Furthermore, the application of machine learning and modern modeling techniques has revolutionized the field, enabling better predictions of drug behavior and interactions.
In essence, the significance of pharmacokinetics extends beyond academic interest; it is a cornerstone of clinical research and drug development. By appreciating the complexities of drug metabolism and the importance of individualized treatment plans, healthcare professionals can optimize therapeutic strategies. This understanding enhances the efficacy of medications and safeguards patient health, emphasizing the need for ongoing education and innovation in pharmacokinetics to improve healthcare outcomes.
What is pharmacokinetics (PK)?
Pharmacokinetics is a branch of pharmacology that studies how the body interacts with a drug over time, focusing on the processes of absorption, distribution, metabolism, and excretion (ADME).
Why is understanding drug metabolism important?
Understanding drug metabolism is essential for determining appropriate medication dosages, predicting drug effects, and ensuring the safety and efficacy of treatments.
How does pharmacokinetics contribute to healthcare?
By examining the pathways medications take within the body, pharmacokinetics helps enhance treatment plans and reduce adverse effects, making it a fundamental aspect of clinical pharmacology that advances healthcare outcomes.