


The article highlights the critical role of preclinical Contract Research Organizations (CROs) in the drug development process, underscoring their significance in evaluating therapeutic candidates prior to clinical trials. Preclinical CROs deliver essential services, including:
These services streamline the drug development process, mitigate risks, and ultimately enhance efficiency, leading to a reduced time to market for new medications. As the landscape of clinical research evolves, the collaboration with preclinical CROs becomes increasingly vital for successful drug development.
The pharmaceutical landscape is undergoing a transformative shift, with early-stage Contract Research Organizations (CROs) emerging as crucial players in the drug development process. By providing specialized services that streamline research and enhance efficiency, these organizations are accelerating the journey from laboratory to clinical trials while ensuring compliance with stringent regulatory standards.
However, as the complexity of drug development increases, pharmaceutical companies face the pressing question: can they effectively navigate the challenges posed by high costs and evolving regulations without the expertise of preclinical CROs?
Early-stage Contract Research Organizations play a pivotal role in the initial phases of medication development, offering specialized services that are essential for pharmaceutical and biotechnology firms. Their primary function is to evaluate new therapeutic candidates prior to clinical trials, which involves conducting laboratory and animal studies to assess safety, efficacy, and pharmacokinetics. By outsourcing these critical tasks, companies gain access to the expertise and advanced resources of preclinical CROs, significantly streamlining their research processes and accelerating the time to market for innovative therapies.
The global preclinical CRO market is expected to surpass USD 6.8 billion by 2025, indicating a strong growth trajectory fueled by the increasing complexity of pharmaceutical development and the growing demand for specialized research services. Notably, approximately 80.8% of pharmaceutical companies are now leveraging preclinical CROs for drug evaluation, highlighting the trend towards outsourcing as a strategic method to enhance efficiency and reduce operational costs.
Expert insights emphasize the importance of preclinical CROs in pharmaceutical research, particularly in navigating the stringent regulatory landscape and ensuring compliance with quality standards. Successful collaborations between pharmaceutical firms and early-stage contract research organizations in the realm of preclinical CROs have demonstrated the advantages of utilizing external expertise, leading to improved project timelines and enhanced research outcomes.
At Bioaccess, we offer a comprehensive range of services, including feasibility studies, site selection, compliance reviews, trial setup, and project management. We prioritize effective reporting on study status, inventory, and adverse events. As the industry evolves, the integration of advanced technologies such as AI and machine learning is anticipated to elevate our services, particularly in bioanalysis and DMPK studies, which are expected to experience significant growth. Nevertheless, challenges such as high costs and complex regulatory environments remain critical factors in this landscape, underscoring the necessity for strategic partnerships with preclinical CROs and early-stage contract research organizations to navigate these challenges effectively.
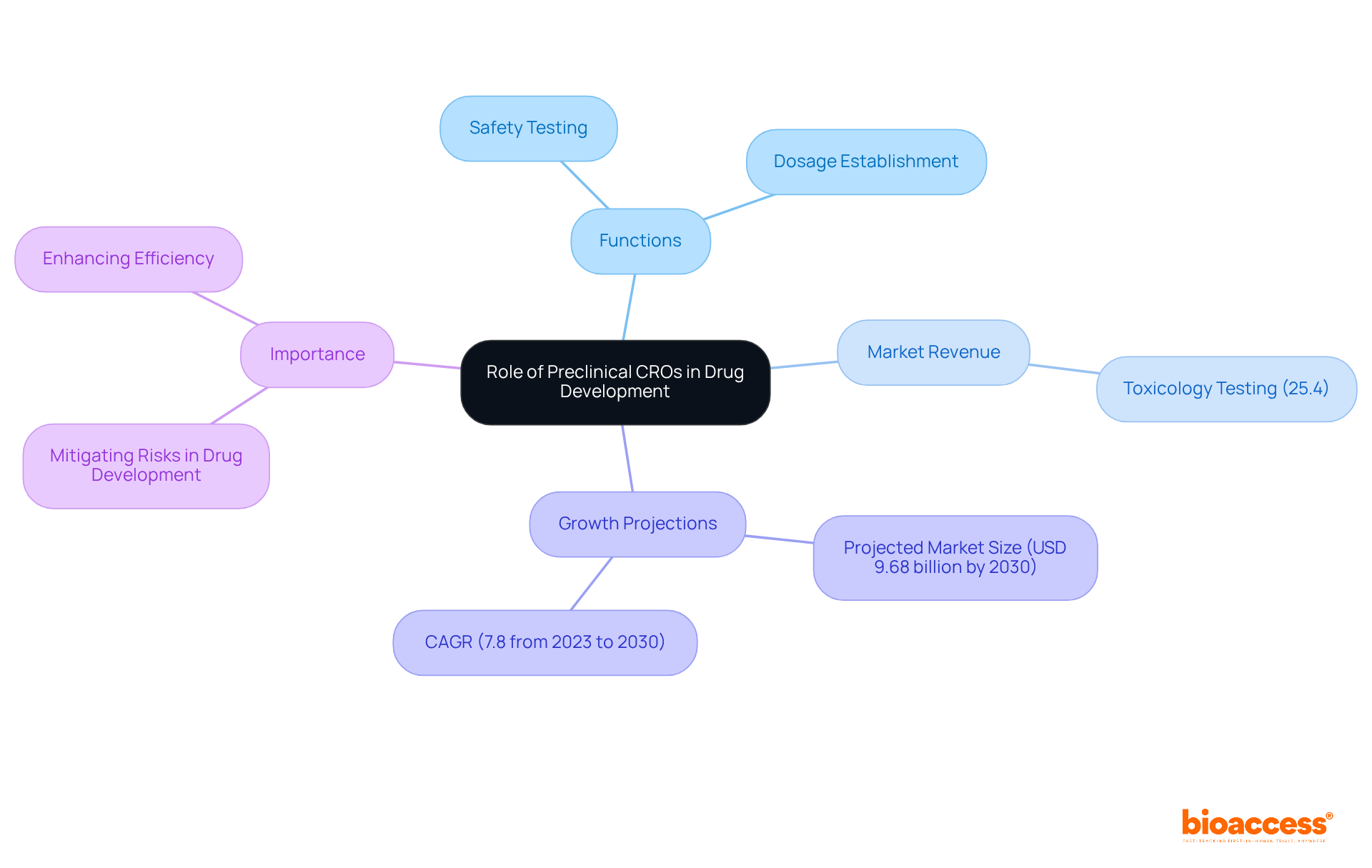
Early-stage contract research organizations are pivotal in the medication creation process, facilitating the transition from laboratory studies to clinical trials. They perform critical preclinical CRO studies that pinpoint potential safety concerns and establish suitable dosages for subsequent clinical testing. For instance, the toxicology testing segment, which accounted for 25.4% of the global preclinical CRO market revenue in 2023, is essential for ensuring medication safety prior to clinical trials. By eliminating less promising candidates, preclinical CROs significantly mitigate risks associated with pharmaceutical development, thereby enhancing overall efficiency.
As the complexity of medication creation increases, the demand for specialized knowledge and resources provided by these organizations has surged, underscoring their importance in navigating the intricate landscape of modern pharmaceuticals. The global preclinical CROs market is projected to reach USD 9.68 billion by 2030, driven by escalating R&D investments and an increasing number of compounds entering preclinical phases. This growth illustrates the vital role that early-phase contract research organizations play in expediting the pharmaceutical development process while ensuring compliance with stringent regulatory requirements.
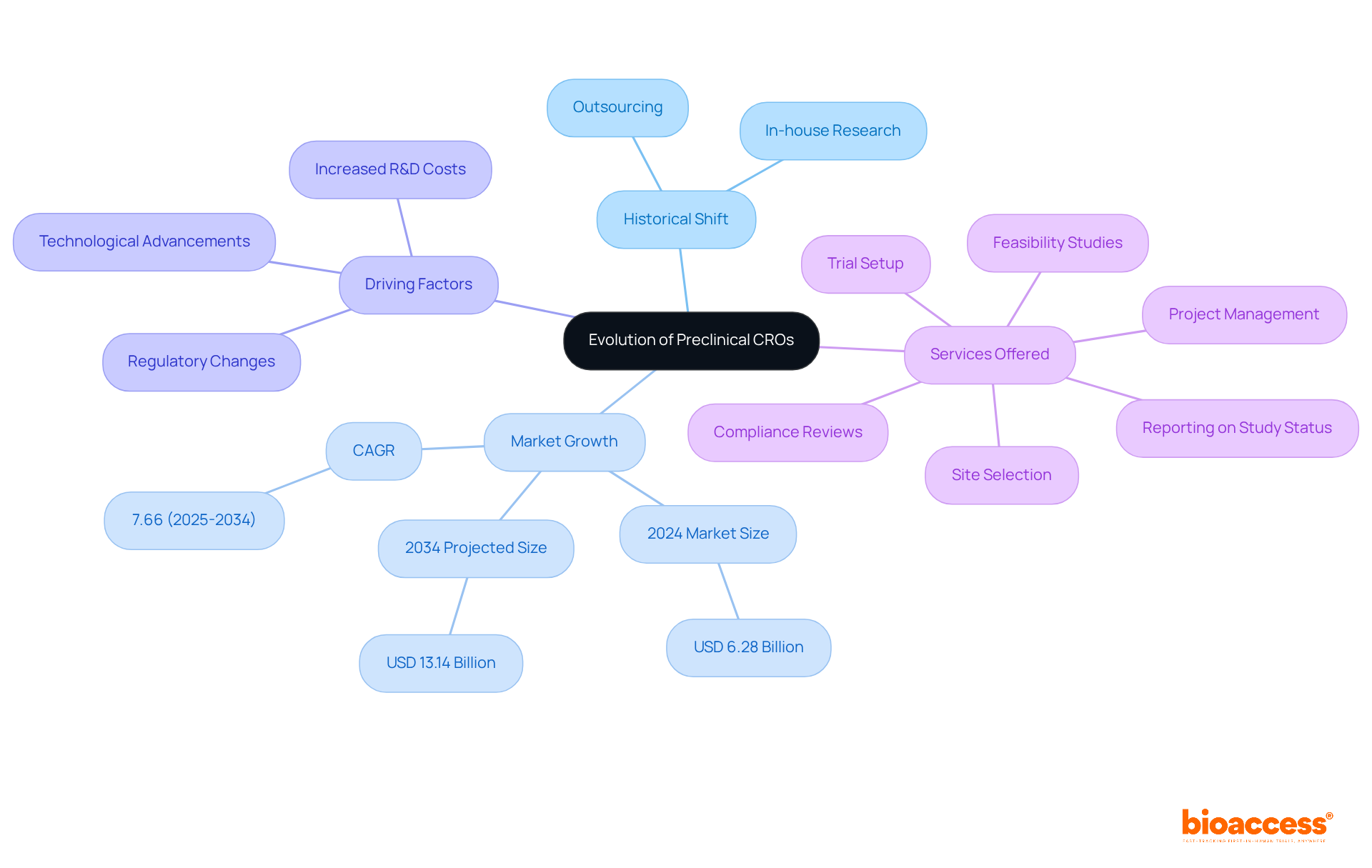
The evolution of Contract Research Organizations (CROs) in their formative years is intricately linked to the increasing complexity of pharmaceutical development and the growing need for specialized expertise. Initially, pharmaceutical companies conducted all research in-house. However, as the industry matured, the pursuit of enhanced efficiency and cost-effectiveness catalyzed a marked shift towards outsourcing. This strategic transition allowed companies to concentrate on their core competencies while leveraging the specialized services offered by early-stage CROs.
In the last decade, the trend of outsourcing has gained significant traction, propelled by escalating R&D costs and the urgency for swift drug development. The preclinical CRO market worldwide, valued at approximately USD 6.28 billion in 2024, is projected to soar to USD 13.14 billion by 2034, reflecting a compound annual growth rate (CAGR) of 7.66% during this timeframe. This remarkable growth is primarily attributed to pharmaceutical companies increasingly outsourcing preclinical CROs to boost efficiency and shorten time to market.
Technological advancements and evolving regulatory landscapes have further reshaped the sector, empowering early-stage CROs to deliver more sophisticated and comprehensive services. These offerings encompass:
For instance, bioaccess provides a meticulous process for advancing medical device trials, ensuring adherence to country-specific requirements while facilitating the selection of research sites and principal investigators. As a result, early-stage CROs have emerged as indispensable partners for pharmaceutical firms, driving the effective progression of new treatments from concept to clinical testing. However, challenges such as regulatory hurdles, competition, recruitment difficulties, and financial constraints may hinder market growth, underscoring the critical need for ongoing investment in workforce development.
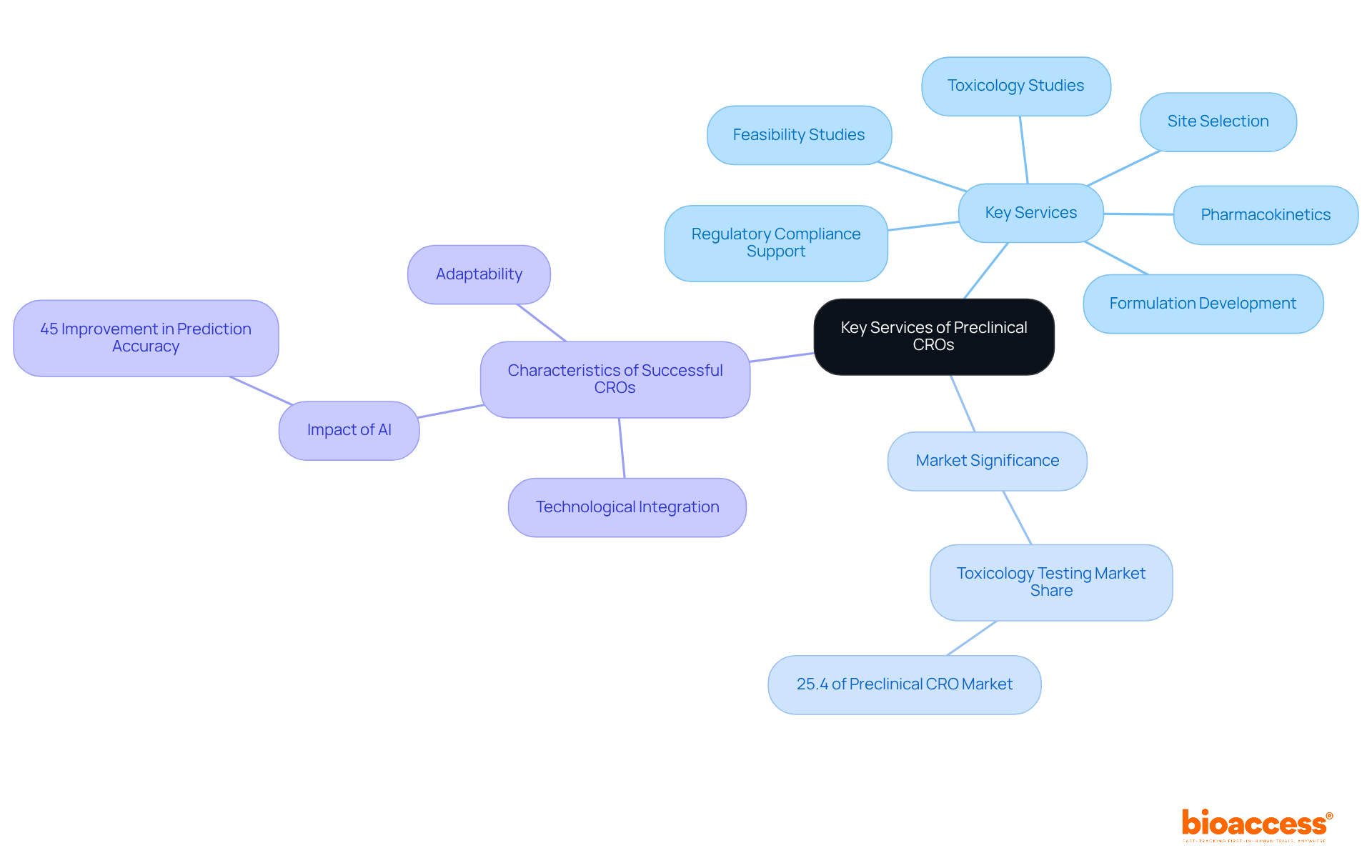
Preclinical CROs play a crucial role in the successful development of new medications by providing a comprehensive array of services. Key services include:
These elements are crucial for navigating the complexities of medication development. Notably, toxicology testing accounted for 25.4% of the preclinical CROs market in 2023, underscoring its significance in ensuring the safety and efficacy of medications.
Furthermore, early-stage contract research organizations conduct both in vitro and in vivo assessments to thoroughly evaluate pharmaceutical candidates. Their project management and consulting services empower clients to make informed decisions throughout the preclinical research process. Successful early-stage contract research organizations are characterized by their ability to deliver high-quality data swiftly, a necessity for maintaining the momentum of pharmaceutical development programs. Expert opinions highlight that adaptability, technological integration, and a strong emphasis on regulatory compliance are defining traits of effective contract research organizations in the early stages. For instance, the integration of AI in preclinical research has enhanced early candidate prediction accuracy by 45%, demonstrating how technological advancements can boost operational efficiency.
The toxicology research conducted by preclinical CROs encompasses evaluations of safety profiles and assessments of potential adverse effects on critical physiological systems. These studies are vital for fulfilling regulatory requirements and ensuring that drug candidates are safe for human trials, ultimately contributing to the success of the drug development pipeline.
The significance of preclinical Contract Research Organizations (CROs) in the drug development landscape is paramount. These specialized entities not only streamline the research process but also enhance the safety and efficacy of new therapeutic candidates prior to their entry into clinical trials. By outsourcing critical tasks to preclinical CROs, pharmaceutical and biotechnology companies can leverage advanced expertise and resources, thereby accelerating the journey of innovative therapies to market.
Throughout this discussion, the pivotal roles played by preclinical CROs have been underscored, including their contributions to:
The burgeoning market for preclinical CROs, projected to reach USD 9.68 billion by 2030, highlights the increasing reliance on these organizations to navigate the complexities of modern pharmaceutical research. Essential services such as:
are crucial for identifying potential safety concerns and ensuring that only the most promising candidates advance to clinical testing.
Reflecting on the broader implications, the collaboration between pharmaceutical companies and preclinical CROs signifies a strategic partnership that fosters innovation and enhances the likelihood of successful drug approval. As the industry continues to evolve, the embrace of advanced technologies and adaptation to regulatory challenges will be vital. The future of drug development hinges on these collaborations, emphasizing the necessity for ongoing investment in preclinical research capabilities to ultimately improve patient outcomes and advance healthcare solutions.
What are preclinical CROs and what is their purpose?
Preclinical Contract Research Organizations (CROs) are specialized entities that play a crucial role in the early stages of medication development. Their primary purpose is to evaluate new therapeutic candidates through laboratory and animal studies to assess safety, efficacy, and pharmacokinetics before clinical trials.
How do pharmaceutical and biotechnology firms benefit from using preclinical CROs?
By outsourcing to preclinical CROs, pharmaceutical and biotechnology firms gain access to specialized expertise and advanced resources, which helps streamline their research processes and accelerate the time to market for new therapies.
What is the projected growth of the global preclinical CRO market?
The global preclinical CRO market is expected to exceed USD 6.8 billion by 2025, driven by the increasing complexity of pharmaceutical development and the rising demand for specialized research services.
What percentage of pharmaceutical companies are utilizing preclinical CROs?
Approximately 80.8% of pharmaceutical companies are leveraging preclinical CROs for drug evaluation, indicating a trend towards outsourcing to enhance efficiency and reduce operational costs.
What are some key services offered by Bioaccess as a preclinical CRO?
Bioaccess offers a comprehensive range of services, including feasibility studies, site selection, compliance reviews, trial setup, and project management, with a focus on effective reporting on study status, inventory, and adverse events.
What technological advancements are expected to influence preclinical CRO services?
The integration of advanced technologies such as AI and machine learning is anticipated to enhance preclinical CRO services, particularly in bioanalysis and DMPK studies.
What challenges do preclinical CROs face in the industry?
Challenges include high costs and complex regulatory environments, which highlight the necessity for strategic partnerships with preclinical CROs to effectively navigate these issues.