


Project FrontRunner is set to transform oncology drug development, addressing a pressing need for more effective treatment pathways. By promoting earlier-stage clinical trials, this initiative not only accelerates access to innovative therapies but also strengthens the quality of evidence regarding their efficacy and safety. As the FDA advocates for a shift in cancer care, stakeholders must consider how they will adapt to this evolving framework. What challenges will they face in unlocking the full potential of this initiative?
FrontRunner is an initiative by the FDA's Oncology Center of Excellence (OCE) that aims to reshape oncology drug development. This program encourages drug sponsors to explore the development and testing of new cancer therapies in earlier treatment settings, particularly for advanced or metastatic diseases. By streamlining the approval process, the project FrontRunner facilitates quicker access to potentially life-saving treatments for individuals.
The initiative underscores the vital role of early-stage clinical studies in oncology, which are essential for generating robust evidence that leads to improved patient outcomes. As we approach 2025, the emphasis on early care environments is increasingly recognized as crucial for enhancing the approval rates of cancer therapies. Specialists assert that engaging in initial-stage studies not only accelerates the development timeline but also provides critical insights into efficacy and safety.
Case studies illustrate the impact of early-stage clinical experiments on cancer therapy approval rates. For instance, the FDA's push for earlier studies has resulted in more comprehensive randomized controlled trials (RCTs), yielding high-quality evidence that enables quicker access to innovative therapies. Furthermore, bioaccess® can significantly bolster these efforts by enrolling treatment-naive cardiology or neurology cohorts 50% faster than traditional Western sites, translating to a $25K savings per patient. This acceleration ensures that the data generated is FDA-ready, effectively addressing the recruitment challenges faced in early-phase clinical trials, particularly for medtech and biopharma startups.
Through these initiatives, FrontRunner is set to be a project frontrunner in greatly enhancing therapeutic options in oncology, ultimately transforming the landscape of cancer care.
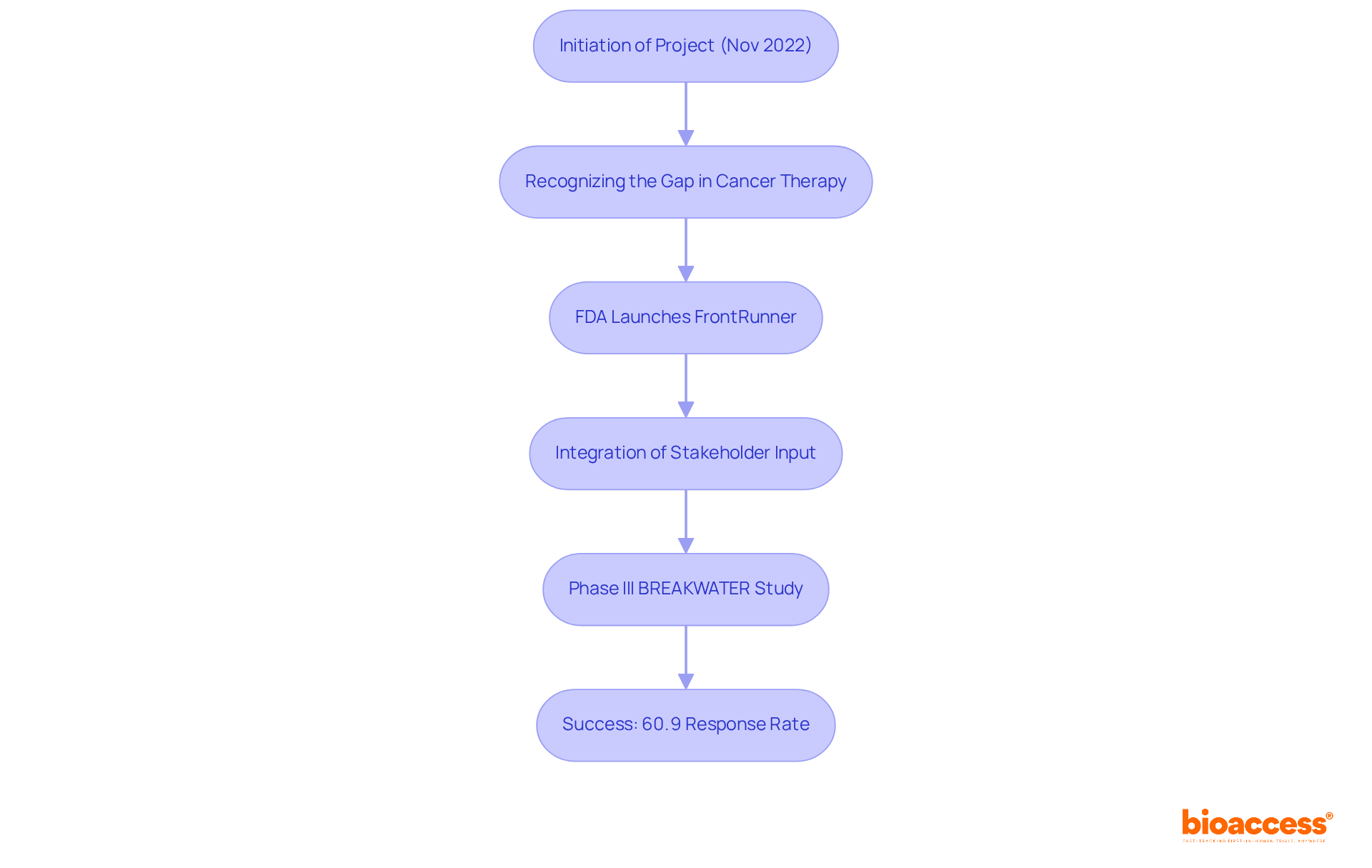
FrontRunner was initiated in November 2022 to address the urgent need for more effective routes in oncology drug development. Traditionally, cancer therapies have been tested primarily in individuals with advanced disease, which often delays access to effective treatments for those in earlier stages. Recognizing this critical gap, the FDA launched FrontRunner to promote earlier clinical trials, facilitating a paradigm shift in how cancer therapies are developed and accessed. This initiative aligns with a broader movement in regulatory science aimed at accelerating the creation of innovative treatments while ensuring safety and effectiveness.
Since its inception, the project FrontRunner has made significant strides by integrating input from a diverse range of stakeholders, including researchers, clinicians, and advocacy organizations. This collaborative approach has refined its strategies, enhancing its potential impact on cancer care. For instance, the Phase III BREAKWATER study, which evaluated a combination of encorafenib, cetuximab, and mFOLFOX6 chemotherapy in individuals with BRAF V600E-mutant metastatic colorectal cancer (mCRC), exemplifies the initiative's influence. The trial reported a 60.9% overall response rate with the three-drug combination, notably surpassing the 40% response rate associated with standard-of-care options. This success not only underscores the effectiveness of innovative treatment combinations but also supports the FDA's expedited approval of new first-line alternatives for patients.
As we approach 2025, the evolution of oncology medication development pathways reflects a commitment to improving outcomes for patients. Experts emphasize the need to adapt regulatory frameworks to keep pace with scientific advancements, ensuring that patients can benefit from the latest therapeutic innovations as quickly as possible. The ongoing development of the project FrontRunner serves as a testament to the FDA's commitment to fostering a more responsive and effective oncology drug development landscape.
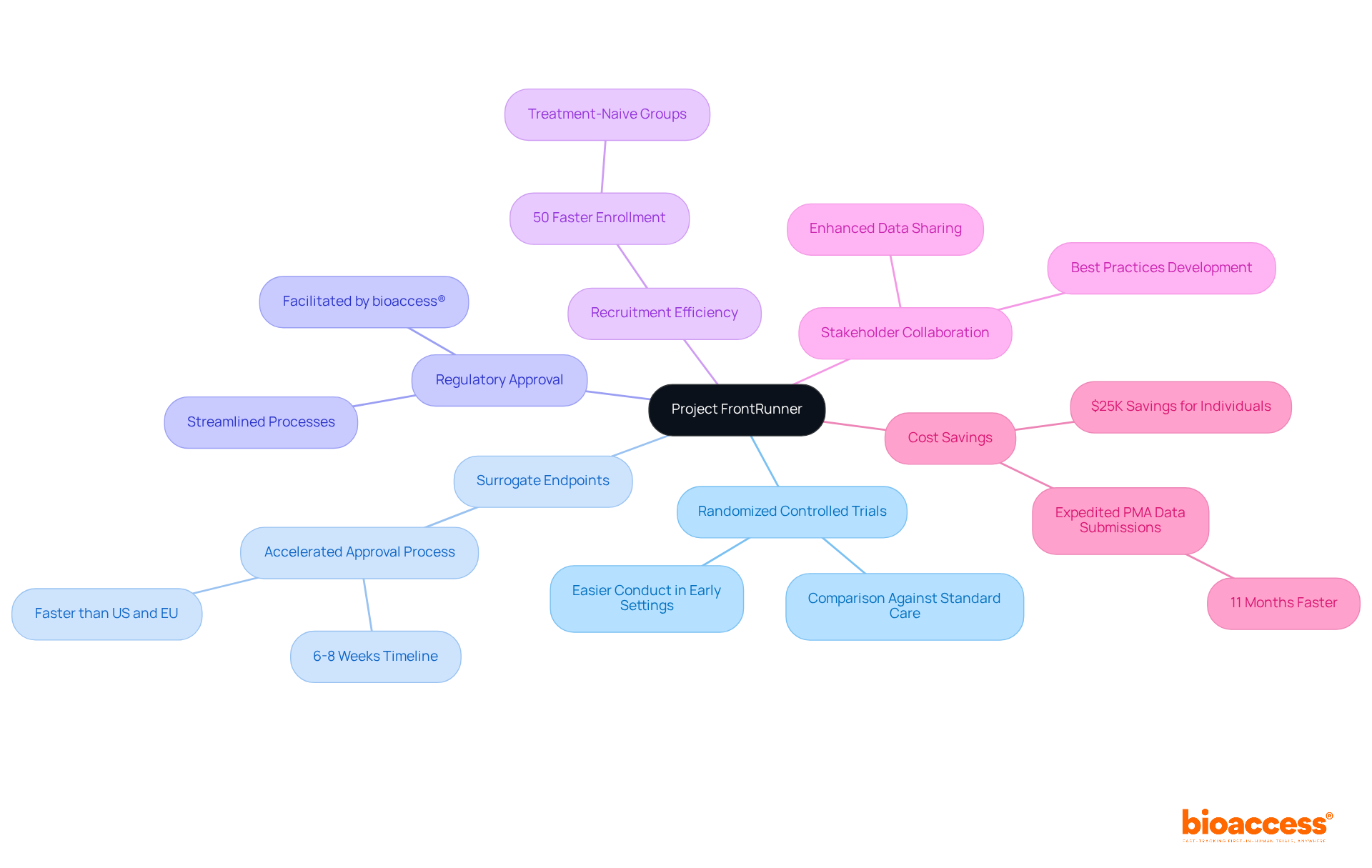
Project Frontrunner stands out for its emphasis on randomized controlled trials (RCTs) in earlier therapeutic lines, enabling a thorough comparison of new therapies against standard care. This initiative not only promotes the use of surrogate endpoints, which can accelerate the approval process by providing earlier indications of treatment efficacy, but it also leverages bioaccess® to facilitate regulatory approval in just 6-8 weeks. This timeline is significantly faster than the typical 6-12 months required in the US and EU, addressing a critical need in the Medtech landscape.
Moreover, this swift approval process, combined with the ability to enroll treatment-naive cardiology or neurology groups 50% quicker than in Western locations, effectively tackles the recruitment challenges often faced by Medtech and biopharma startups. Project Frontrunner also fosters collaboration among stakeholders, including drug developers, regulatory bodies, and clinical researchers, to enhance data sharing and best practices. This cooperative approach aims to elevate the overall standard of clinical studies, ensuring that new treatments are both safe and effective for patients.
In addition to these advancements, the initiative is designed to realize $25K in savings for individuals and expedite PMA data submissions by 11 months, further streamlining clinical study processes. The importance of collaboration in this context cannot be overstated; it is essential for overcoming the hurdles in clinical research and achieving successful outcomes.
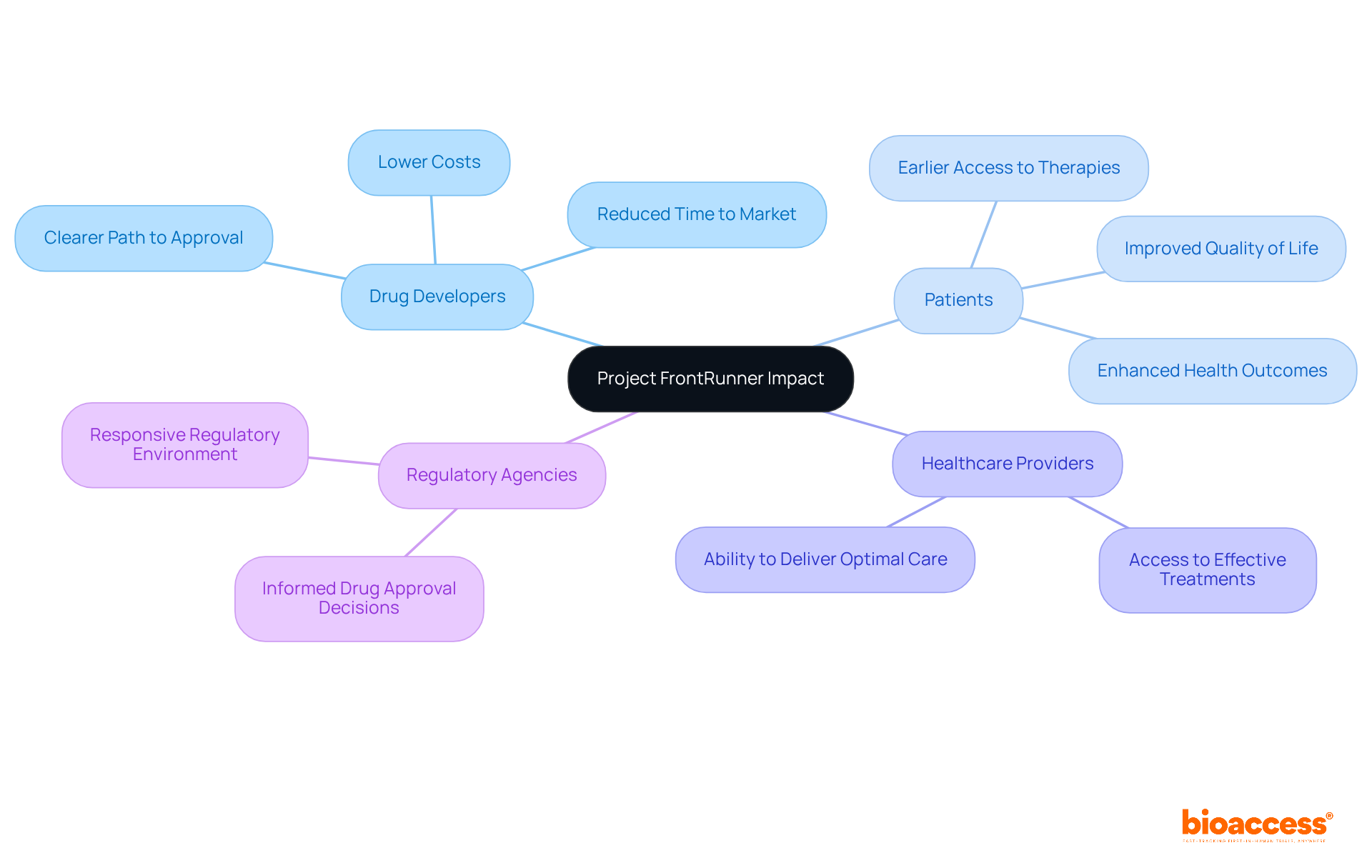
Project Frontrunner significantly impacts the clinical research ecosystem, benefiting a diverse range of stakeholders. For drug developers, this initiative paves a clearer path to approval, potentially slashing the time and costs tied to bringing new therapies to market. bioaccess® plays a crucial role in this process by offering comprehensive clinical trial management services. These include:
Patients are poised to gain earlier access to groundbreaking therapies, which can greatly enhance their quality of life and health outcomes. Healthcare providers will also benefit, as they gain access to more effective treatment options sooner, allowing them to deliver optimal care. Regulatory agencies, such as the FDA, can utilize data generated from earlier trials to make informed decisions about drug approvals, fostering a more responsive and adaptive regulatory environment.
As the biologics market is projected to grow at a compound annual growth rate of 15% until 2027, the importance of initiatives like FrontRunner becomes increasingly evident. This growth highlights the necessity for innovative strategies to tackle challenges in drug development, particularly concerning monoclonal antibodies (mAbs). Overall, the project Frontrunner embodies a collaborative effort aimed at enhancing the efficiency and effectiveness of oncology drug development, with the potential to transform patient care in cancer treatment.
As R.S. Grey aptly stated, 'She believed she could, so she did,' reflecting the determination required to navigate this evolving landscape.
Project FrontRunner stands as a pivotal initiative from the FDA's Oncology Center of Excellence, set to redefine oncology drug development. By prioritizing early-stage clinical trials, this program accelerates the approval process for new cancer therapies, ensuring that patients have timely access to potentially life-saving treatments. This commitment to reshaping cancer therapy development highlights the critical role of innovation in enhancing patient outcomes.
Key insights from the discussion reveal that Project FrontRunner:
Its emphasis on early intervention and streamlined approval processes showcases its potential to significantly enhance the efficiency of drug development, ultimately benefiting patients, healthcare providers, and regulatory bodies alike.
Looking at the broader implications, advancing initiatives like Project FrontRunner is essential as the biologics market continues to expand. The collaborative efforts promoted by this initiative not only elevate the standards of clinical studies but also pave the way for innovative solutions that can transform cancer care. Embracing such forward-thinking approaches is vital for overcoming the challenges in oncology drug development, ensuring that patients receive the highest quality care in their battle against cancer.
What is Project FrontRunner?
Project FrontRunner is an initiative by the FDA's Oncology Center of Excellence (OCE) that aims to reshape oncology drug development by encouraging drug sponsors to explore the development and testing of new cancer therapies in earlier treatment settings, particularly for advanced or metastatic diseases.
What is the main goal of Project FrontRunner?
The main goal of Project FrontRunner is to streamline the approval process for cancer therapies, facilitating quicker access to potentially life-saving treatments for individuals.
Why are early-stage clinical studies important in oncology?
Early-stage clinical studies are vital as they generate robust evidence that leads to improved patient outcomes. They are increasingly recognized as crucial for enhancing the approval rates of cancer therapies.
How does Project FrontRunner impact the timeline for drug development?
Engaging in initial-stage studies accelerates the development timeline and provides critical insights into the efficacy and safety of cancer therapies.
What evidence supports the effectiveness of early-stage clinical experiments?
Case studies have shown that the FDA's push for earlier studies has resulted in more comprehensive randomized controlled trials (RCTs), yielding high-quality evidence that enables quicker access to innovative therapies.
How does bioaccess® contribute to the efforts of Project FrontRunner?
Bioaccess® can significantly enhance these efforts by enrolling treatment-naive cardiology or neurology cohorts 50% faster than traditional Western sites, resulting in a $25K savings per patient and ensuring that the data generated is FDA-ready.
What challenges does Project FrontRunner address in early-phase clinical trials?
Project FrontRunner addresses recruitment challenges faced in early-phase clinical trials, particularly for medtech and biopharma startups, by streamlining processes and improving enrollment efficiency.
What is the overall impact of Project FrontRunner on cancer care?
Project FrontRunner is set to enhance therapeutic options in oncology, ultimately transforming the landscape of cancer care by facilitating quicker access to effective treatments.