


The article emphasizes the critical components and significance of Quality Management Systems (QMS) for medical devices, underlining their essential role in ensuring product quality and regulatory compliance. A well-implemented QMS is indispensable for minimizing risks, enhancing patient safety, and improving overall operational efficiency. Evidence shows that organizations with robust QMS protocols experience reduced product recalls and demonstrate compliance with global standards such as ISO 13485 and FDA regulations. This underscores the necessity for medical device manufacturers to prioritize the establishment of effective QMS, thereby fostering trust and reliability in their products.
Understanding the intricacies of Quality Management Systems (QMS) for medical devices is essential in an industry where patient safety and regulatory compliance are paramount. A well-structured QMS not only guarantees that healthcare products consistently meet stringent quality standards but also enhances operational efficiencies and fosters a culture of continuous improvement. As the landscape of medical device regulations evolves, organizations face the pressing challenge of adapting to new standards while mitigating risks associated with product development and market entry.
What are the key components of an effective QMS, and how can they transform the medical device manufacturing process?
A QMS medical device for healthcare products serves as a structured framework that meticulously records processes, procedures, and responsibilities essential for achieving quality policies and objectives. This system encompasses the entire lifecycle of a healthcare instrument, from design and development to production, distribution, and post-market monitoring. The primary objective of a QMS medical device is to ensure that healthcare products consistently meet both customer and regulatory standards, thereby enhancing patient safety and product effectiveness.
In 2024, the Medical Device Management System market was valued at approximately 3.5 billion USD and is projected to grow to 7.2 billion USD by 2033. This significant increase underscores a heightened focus on management within the industry. A well-executed QMS medical device is essential for ensuring compliance with global standards such as ISO 13485, which outlines specific criteria for quality management systems in the healthcare sector. Moreover, a comprehensive QMS medical device facilitates vital services, including:
This ensures meticulous management of all clinical trial aspects.
The role of INVIMA, recognized as a Level 4 health authority by PAHO/WHO, is pivotal in overseeing device regulations, further highlighting the necessity of compliance within the QMS framework. As the healthcare landscape evolves, the application and evaluation of the QMS medical device have become indispensable for ensuring optimal patient care, minimizing healthcare errors, and streamlining operations within healthcare facilities. Understanding the statistical trends surrounding these systems offers invaluable insights into their effectiveness, influencing funding, regulations, and technological advancements in the healthcare industry. According to Steve Bennett, 'the implementation and analysis of healthcare management systems, including QMS medical device, have never been more crucial,' reinforcing their significance in today’s health environment.
The significance of a QMS medical device in development is paramount. It establishes a structured framework that ensures all facets of the development process are meticulously controlled and documented, which is vital for meeting regulatory requirements such as EU MDR, FDA 21 CFR Part 820, and ISO 13485:2016. A robust QMS enables organizations to proactively identify and mitigate risks during the early stages of development, significantly decreasing the chances of expensive recalls or regulatory sanctions. For instance, companies that have implemented effective QMS practices have reported a notable reduction in product recalls, showcasing the system's effectiveness in enhancing product reliability. The case study titled "The Right QMS Makes All the Difference" illustrates how a well-designed QMS can streamline processes and reduce recalls, emphasizing its critical role in the industry.
Moreover, a well-implemented QMS cultivates a culture of continuous improvement, allowing organizations to refine their processes and products over time. This dedication to excellence not only results in better patient outcomes but also strengthens the organization’s reputation within the competitive marketplace. Experts such as Dan Holton stress that excellence can drive enterprise value and should be seen as a culture, not merely a function. Incorporating excellence management into the heart of healthcare product development is vital for promoting innovation while guaranteeing safety and effectiveness. Ultimately, the cost reductions linked to adopting a QMS medical device can be significant, as organizations face fewer compliance challenges and experience improved operational efficiencies, making it a crucial investment for any healthcare company. Insights from David Narrow emphasize that companies exhibiting a strong culture of excellence tend to attain higher valuations, further highlighting the financial advantages of a well-implemented QMS.
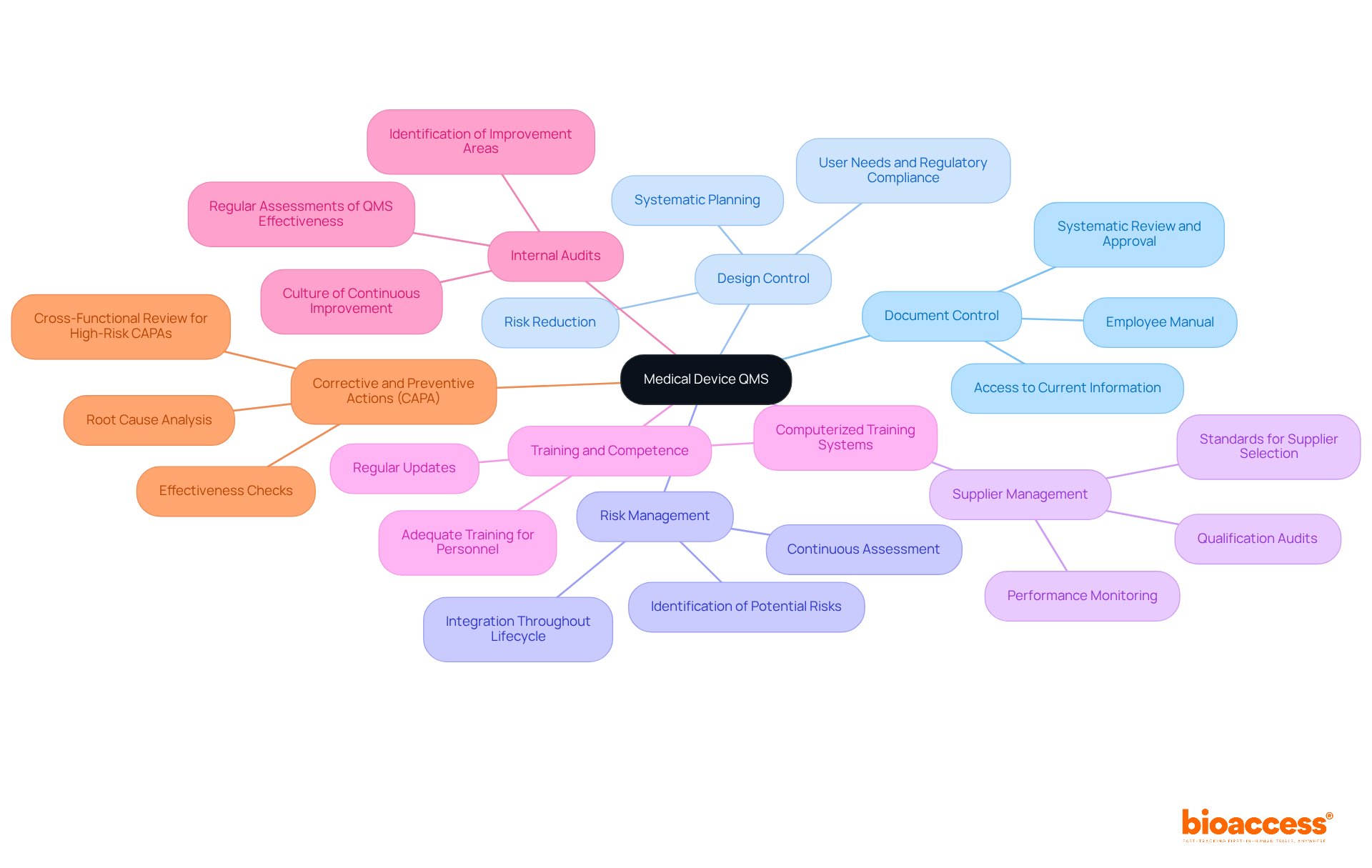
Key components of a qms medical device are essential for ensuring compliance and quality standards.
Document Control is a critical process that guarantees all documents are systematically reviewed, approved, and updated as necessary. This not only helps organizations avoid costly errors but also ensures that all personnel have access to the most current information, with an employee manual providing vital instruction on company expectations.
Design Control involves systematic planning and documentation of the design process, ensuring that the product meets user needs and regulatory requirements. A robust design control process significantly reduces the risk of non-compliance and enhances product safety.
Risk Management is vital for identifying potential risks associated with the device and implementing strategies to mitigate them. According to ISO 14971, risk management should be integrated throughout the product lifecycle, allowing for continuous assessment and management of risks. This integration is crucial for timely updates to the Risk Management File (RMF) based on newly identified risks post-release.
Supplier Management establishes standards for choosing and assessing suppliers, ensuring that procured materials and services meet excellence benchmarks. This includes conducting qualification audits and performance monitoring to maintain high-quality inputs, with key components such as qualification audits, technical agreements, and performance monitoring.
Training and competence are essential for ensuring that all personnel involved in the qms medical device are adequately trained and skilled in their roles. Regular training updates keep staff informed about the latest practices and regulatory requirements. Organizations increasingly adopt computerized training systems for onboarding new employees, saving time and money.
Internal Audits involve regular assessments of QMS effectiveness, identifying areas for improvement. These audits are crucial for ensuring compliance and fostering a culture of continuous improvement within the organization. An internal audit program serves as a robust defense against external findings.
Corrective and Preventive Actions (CAPA) address non-conformities and prevent their recurrence, essential for maintaining product standards and safety. A well-defined CAPA process begins with thorough root cause analysis and includes effectiveness checks to ensure that corrective actions are successful. High-risk CAPAs should undergo cross-functional review before closure, ideally involving assurance, engineering, and regulatory teams.
These components collaborate to form a unified system that aids in the development and upkeep of high-quality healthcare products, incorporating qms medical device principles, ultimately improving patient safety and adherence to regulatory standards. The worldwide healthcare management software market is anticipated to expand considerably, with the cloud segment predicted to show the highest CAGR of 17.39% during the forecast period, underscoring the growing significance of efficient QMS in the healthcare sector.
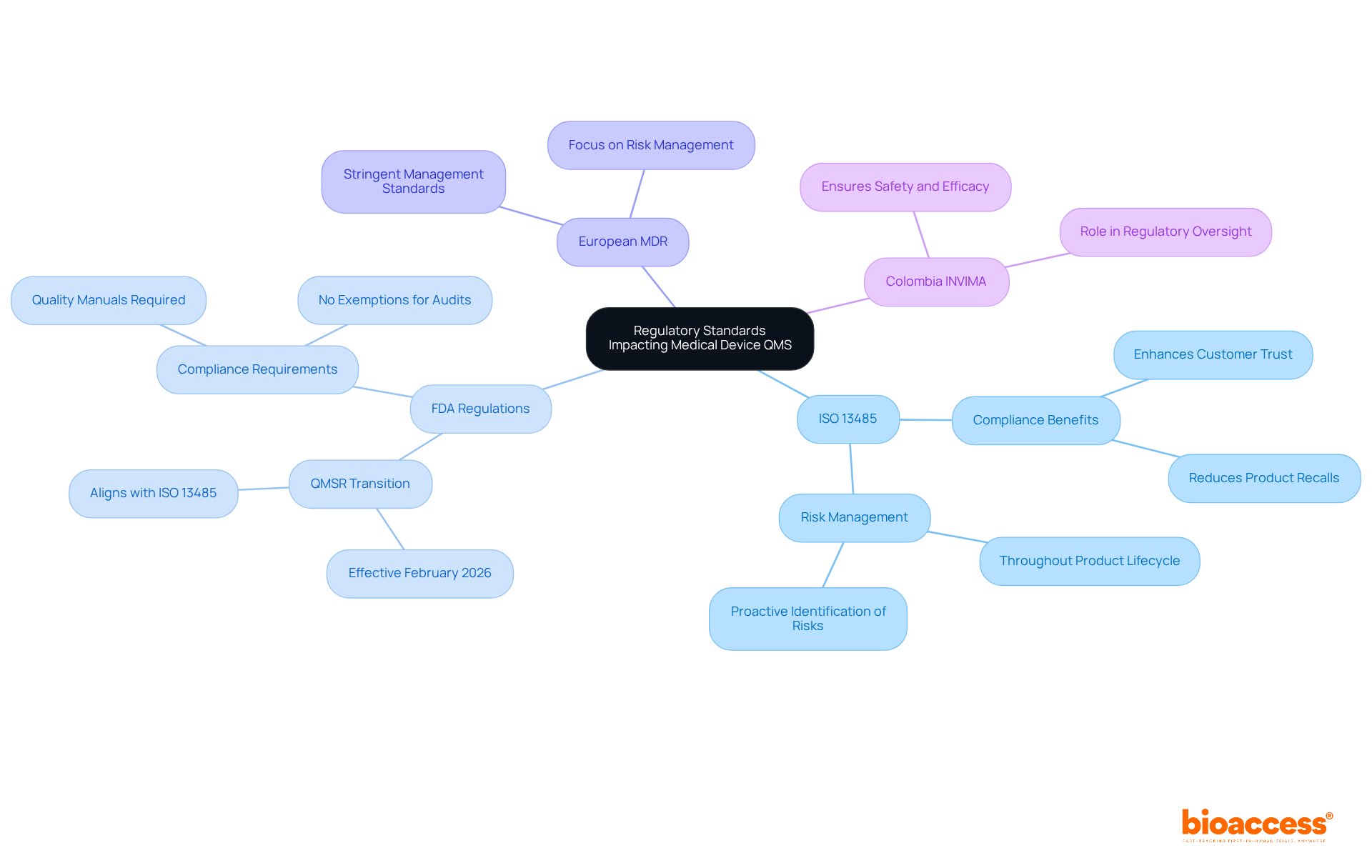
Regulatory standards are crucial in establishing the framework for QMS medical device systems in the healthcare equipment sector. Key standards include:
ISO 13485: This global standard specifies the criteria for a management system, ensuring organizations can consistently provide healthcare products and associated services that fulfill both customer and regulatory expectations. Compliance with ISO 13485 is increasingly recognized as a means to enhance customer retention and satisfaction, as it demonstrates a commitment to high-quality products.
FDA Regulations: In the U.S., the Food and Drug Administration (FDA) mandates that medical device manufacturers establish and maintain a QMS compliant with the Quality System Regulation (QSR). As of February 2026, the FDA's new QMS medical device Regulation (QMSR) will replace the QSR, requiring manufacturers to adapt to updated compliance requirements. This transition emphasizes the importance of proactive risk management throughout the product lifecycle, aligning closely with ISO 13485 principles. The FDA has also indicated that standards manuals are now a necessity under the QMSR, which was not the situation under the QSR alone.
European Medical Device Regulation (MDR): In Europe, the MDR enforces stringent management standards, concentrating on effective risk management and post-market surveillance. Compliance with these regulations is not merely a legal obligation; it is a best practice that significantly enhances product quality and patient safety.
In Colombia, the regulatory framework is managed by INVIMA (Colombia National Food and Drug Surveillance Institute), which plays an essential role in ensuring that healthcare instruments meet safety and efficacy standards. INVIMA's designation as a Level 4 health authority by PAHO/WHO highlights its proficiency in overseeing health products, including healthcare tools. Experts like Ana Criado, Director of Regulatory Affairs, and Katherine Ruiz, an expert in Regulatory Affairs for Medical Devices and In Vitro Diagnostics, contribute significantly to navigating these complex regulations.
The integration of these standards into a QMS medical device fosters a culture of continual improvement, which enables organizations to effectively identify and mitigate operational risks. For instance, companies that have successfully implemented ISO 13485 have reported fewer product recalls and enhanced customer trust, demonstrating the tangible benefits of adhering to these standards. Additionally, with faulty healthcare instruments leading to around 200,000 injuries annually, the essential requirement for adherence to ISO 13485 and FDA regulations cannot be emphasized enough. As the landscape of medical device regulation evolves, staying informed and compliant with these standards is crucial for manufacturers aiming to ensure the safety and efficacy of their products.
Understanding the significance of Quality Management Systems (QMS) for medical devices is crucial in ensuring that healthcare products not only meet regulatory requirements but also prioritize patient safety. A robust QMS serves as a backbone for organizations, enabling them to streamline processes, enhance product reliability, and foster a culture of continuous improvement. As the medical device landscape continues to evolve, the importance of implementing an effective QMS cannot be overstated.
The article outlines the key components of a medical device QMS, including:
Each of these elements plays a vital role in maintaining compliance with regulatory standards such as ISO 13485, FDA regulations, and European Medical Device Regulations. By integrating these components into their operations, organizations can mitigate risks, reduce product recalls, and ultimately improve patient outcomes.
As the healthcare industry faces increasing scrutiny and regulatory challenges, it is imperative for medical device manufacturers to prioritize the implementation of a comprehensive QMS. This not only enhances operational efficiencies but also positions organizations for long-term success in a competitive market. Embracing the principles of quality management is not just a regulatory obligation; it is a commitment to excellence that can lead to safer, more effective healthcare products.
What is a Quality Management System (QMS) for medical devices?
A QMS for medical devices is a structured framework that records processes, procedures, and responsibilities essential for achieving quality policies and objectives throughout the lifecycle of a healthcare product, from design and development to production, distribution, and post-market monitoring.
What is the primary objective of a QMS in the medical device industry?
The primary objective of a QMS in the medical device industry is to ensure that healthcare products consistently meet customer and regulatory standards, thereby enhancing patient safety and product effectiveness.
What was the market value of the Medical Device Management System in 2024, and what is it projected to be by 2033?
The Medical Device Management System market was valued at approximately 3.5 billion USD in 2024 and is projected to grow to 7.2 billion USD by 2033.
Why is compliance with global standards like ISO 13485 important for QMS?
Compliance with global standards such as ISO 13485 is essential for ensuring that quality management systems in the healthcare sector meet specific criteria, which helps maintain product quality and safety.
What services does a comprehensive QMS medical device facilitate?
A comprehensive QMS medical device facilitates services such as feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting on serious and non-serious adverse events.
What role does INVIMA play in the context of QMS for medical devices?
INVIMA, recognized as a Level 4 health authority by PAHO/WHO, plays a pivotal role in overseeing device regulations, highlighting the necessity of compliance within the QMS framework.
How does the application of QMS in healthcare evolve?
The application and evaluation of QMS in healthcare have become indispensable for ensuring optimal patient care, minimizing healthcare errors, and streamlining operations within healthcare facilities.
What insights can be gained from statistical trends surrounding QMS?
Understanding statistical trends surrounding QMS offers insights into their effectiveness, influencing funding, regulations, and technological advancements in the healthcare industry.