


Regulatory affairs in the pharmaceutical sector are paramount for ensuring compliance with safety, efficacy, and quality standards. This not only protects public health but also facilitates the market introduction of new therapies.
Regulatory professionals play a critical role in this landscape, including:
Their efforts safeguard patients and enhance the efficiency of clinical trials, underscoring the significance of their contributions in advancing clinical research.
Regulatory affairs in the pharmaceutical industry are the backbone of compliance, ensuring that every drug developed meets rigorous safety and efficacy standards. As the healthcare landscape evolves, the role of regulatory professionals becomes increasingly significant, navigating complex regulations to facilitate the introduction of innovative therapies.
With rising compliance demands and intricate legal frameworks, leaders face the critical challenge of managing these complexities while safeguarding public health. How can they effectively address these challenges and ensure the integrity of the healthcare system?
Regulatory affairs in pharma are a critical field dedicated to ensuring compliance with the myriad laws and regulations governing the development, testing, manufacturing, and marketing of pharmaceutical products. This multifaceted domain encompasses essential tasks such as the preparation and submission of compliance documents, effective communication with governing agencies, and the oversight of clinical trials. Compliance specialists serve as a vital link between pharmaceutical firms and regulatory bodies, ensuring that products meet stringent safety, efficacy, and quality standards prior to market entry.
Key oversight roles involve:
Effective oversight strategies often incorporate proactive engagement with governing entities, leveraging insights from market trends, and utilizing advanced tools like AI to streamline processes and enhance compliance.
The importance of compliance management is paramount; it is essential for maintaining pharmaceutical adherence and safeguarding public health. As the industry faces increasing compliance demands and complexities, the role of professionals in regulatory affairs in pharma becomes even more critical. Their expertise not only aids in navigating the intricate legal landscape but also accelerates the introduction of innovative therapies to the market, ultimately benefiting patients and healthcare systems alike.
For medical device startups, navigating this process can be particularly daunting due to compliance hurdles, competitive pressures, recruitment challenges, and financial constraints. Companies like bioaccess® are addressing these issues by providing a comprehensive approach to advancing medical device studies, which encompasses:
In Colombia, securing clinical study approval necessitates navigating the requirements of the IRB/EC, INVIMA, and MinCIT import permits. bioaccess® expedites this process, enabling approval in as little as 6-8 weeks, in contrast to the typical 6-12 months required in the US/EU. Their expertise not only simplifies navigation through the complex legal framework but also facilitates the rapid introduction of innovative therapies to the market, ultimately benefiting patients and healthcare systems alike. As the industry faces escalating compliance demands and complexities, the role of professionals in regulatory affairs in pharma becomes increasingly vital, particularly in enhancing the efficiency of accelerated clinical trials and patient enrollment for Medtech and Biopharma startups.
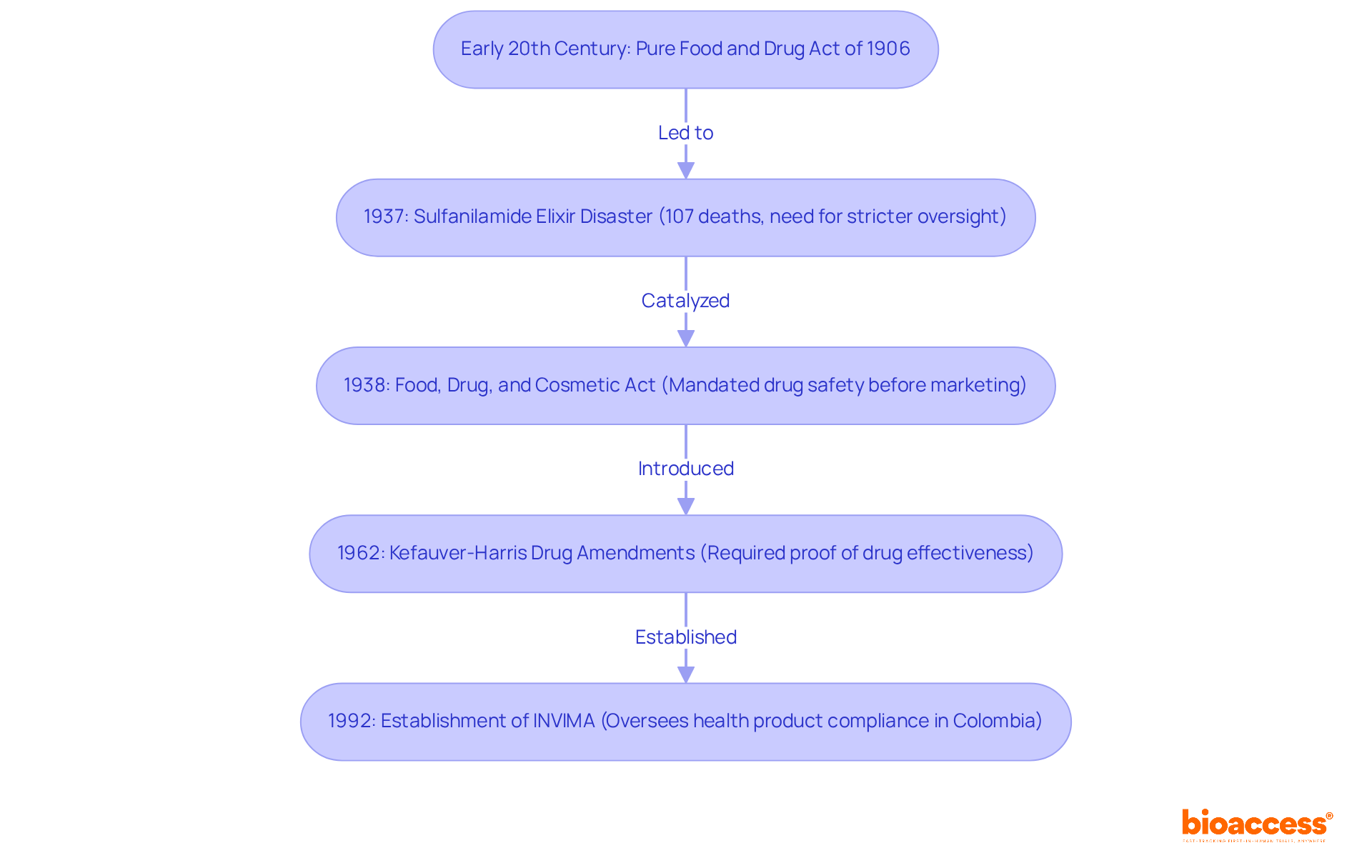
The evolution of compliance matters within the pharmaceutical industry traces back to the early 20th century, marked by the enactment of the Pure Food and Drug Act of 1906 in the United States. This pivotal legislation sought to eradicate the sale of misbranded or adulterated foods and drugs, laying the groundwork for future oversight frameworks. The devastating sulfanilamide elixir disaster in 1937, which claimed the lives of 107 individuals, highlighted the urgent need for stricter oversight and catalyzed the introduction of the Food, Drug, and Cosmetic Act of 1938. This act mandated that new drugs be proven safe prior to marketing, significantly reshaping the oversight landscape.
Over the decades, the field has evolved in response to scientific advancements and technological progress, alongside an increasing public demand for transparency and safety in drug development. Key milestones, such as the Kefauver-Harris Drug Amendments of 1962, which required producers to demonstrate drug effectiveness, further reinforced the role of oversight in safeguarding public health.
In Colombia, the governance framework is overseen by INVIMA (Instituto Nacional de Vigilancia de Medicamentos y Alimentos), established in 1992 under the Ministry of Health and Social Protection. INVIMA is responsible for examining and supervising the marketing and production of health products, ensuring compliance with health standards, and granting medical approval for the import and export of products. The Directorate for Medical Devices and other Technologies within INVIMA plays a vital role in monitoring medical devices and managing pre- and post-market programs.
Recognized as a Level 4 health authority by the Pan American Health Organization/World Health Organization, INVIMA's classification reflects its capability in executing health regulation functions to ensure the safety, efficacy, and quality of medicines. The ongoing development of compliance matters within regulatory affairs in pharma is continually influenced by globalization, technological advancements, and the increasing complexity of drug creation processes, necessitating a robust framework to safeguard patient safety and effectiveness.
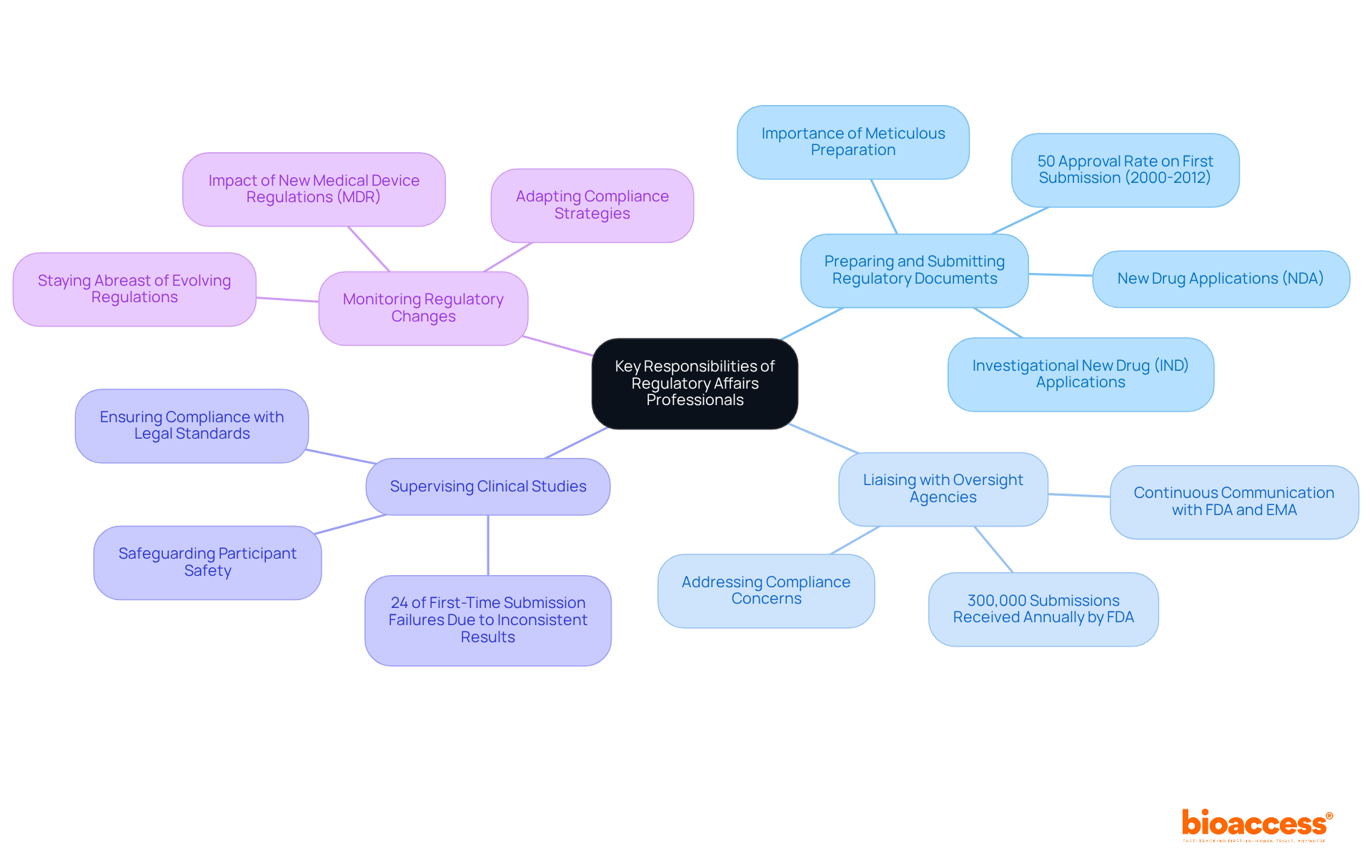
In the pharmaceutical sector, regulatory affairs in pharma are pivotal, with regulatory specialists playing a crucial role in ensuring adherence to regulations and facilitating the drug approval process. Their key functions include:
Preparing and Submitting Regulatory Documents: These professionals compile and submit critical documentation for drug approvals, such as Investigational New Drug (IND) applications and New Drug Applications (NDA). The quality of these submissions is paramount; studies indicate that 50% of new drug applications were approved upon first submission to the FDA between 2000 and 2012, underscoring the necessity of meticulous preparation.
Liaising with oversight agencies: They maintain continuous communication with oversight bodies like the FDA and EMA, addressing concerns and ensuring compliance with established guidelines in regulatory affairs in pharma. This interaction is crucial, as the FDA receives over 300,000 submissions annually, necessitating clear and effective communication to navigate the intricacies of the approval process.
Supervising Clinical Studies: Compliance specialists ensure that clinical studies adhere to legal standards, safeguarding participant safety and data accuracy. Their oversight is vital, particularly given that 24% of first-time submission failures are associated with inconsistent study results across endpoints or locations, which can jeopardize study outcomes. Organizations like bioaccess® significantly contribute to this process by providing services such as patient recruitment, compliance approval, and trial data delivery, thereby enhancing the efficiency of clinical trials in Latin America.
Monitoring regulatory changes: This is an essential responsibility within regulatory affairs in pharma, as it requires staying abreast of evolving regulations and guidelines. This vigilance enables compliance specialists to adapt strategies as necessary, ensuring ongoing adherence. For example, the introduction of the new Medical Device Regulations (MDR) in the EU has led to extended approval times, emphasizing the need for proactive compliance strategies.
In conclusion, the role of regulatory professionals is critical to the success of clinical studies and the timely approval of new treatments, as evidenced by various case studies that illustrate the impact of effective regulatory oversight on submission outcomes. Leveraging the expertise of organizations like bioaccess® can significantly enhance market access and streamline the clinical trial process.
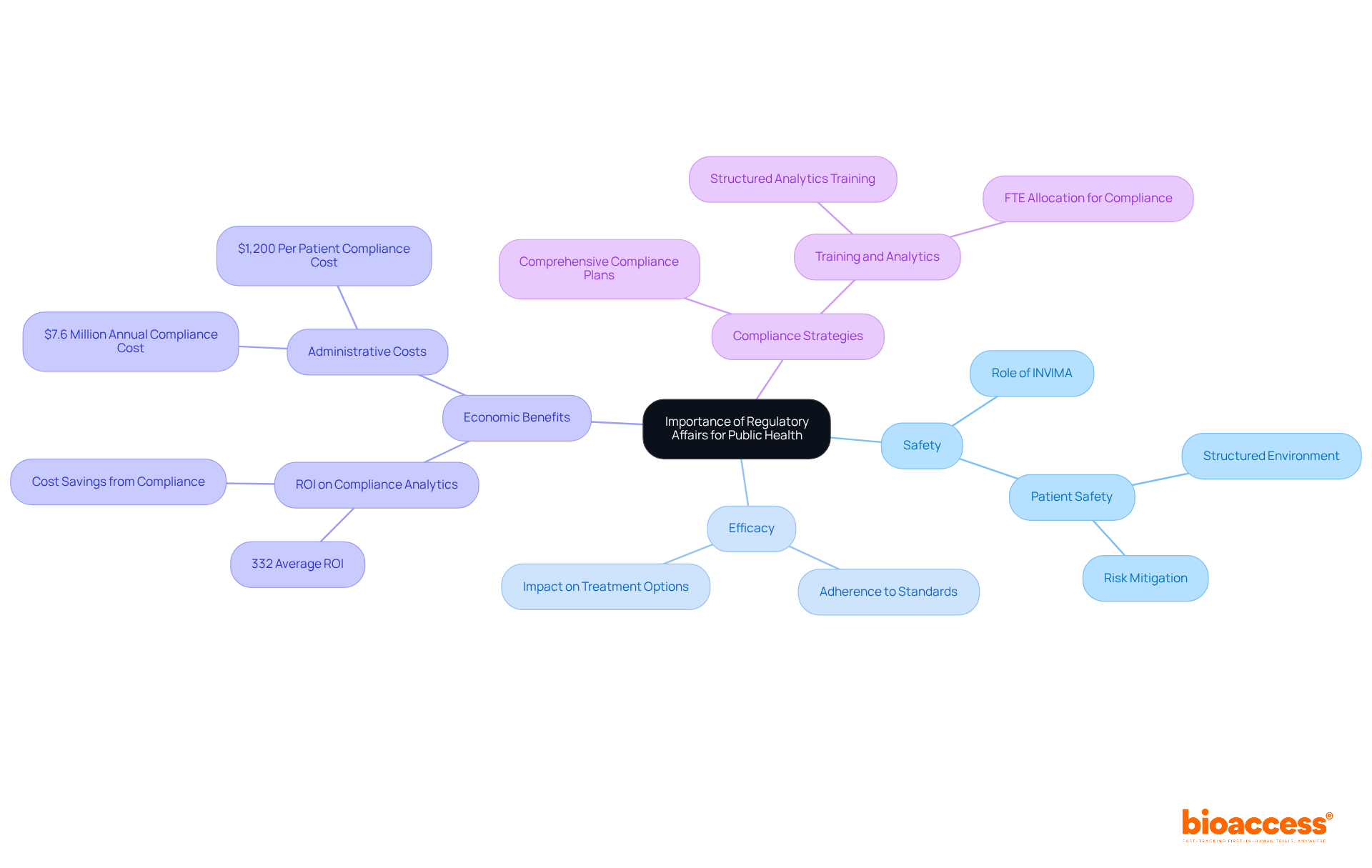
Regulatory affairs in pharma are crucial in safeguarding public health by ensuring that pharmaceutical products meet stringent safety, efficacy, and quality standards. By enforcing adherence to these rigorous regulations, professionals in this field prevent harmful products from entering the market, thereby protecting patients and consumers alike. This unwavering commitment to regulatory compliance not only facilitates timely access to innovative treatments but also fosters public confidence in the healthcare system.
In Colombia, for instance, the INVIMA (Colombia National Food and Drug Surveillance Institute) oversees the regulatory framework, ensuring that health products conform to established standards. Recognized as a Level 4 health authority by the Pan American Health Organization/World Health Organization, INVIMA plays a pivotal role in monitoring and controlling medical devices, which is essential for maintaining public safety.
Healthcare organizations that prioritize adherence can achieve significant enhancements in patient safety and operational efficiency. For example, hospitals investing in compliance analytics often see a return on investment averaging 332% within three years, underscoring the economic benefits of a robust adherence strategy. Furthermore, compliance with established standards creates a structured environment that enhances accountability and mitigates risks, ultimately laying the groundwork for patient safety.
As the landscape of medical technology and pharmaceuticals evolves rapidly, the importance of compliance affairs becomes increasingly vital in balancing the need for innovation with the imperatives of safety and efficacy. This dynamic emphasizes the necessity of developing comprehensive adherence strategies that encompass local, national, and international guidelines, enabling healthcare providers to navigate the complexities of legal requirements effectively. Additionally, healthcare providers contend with approximately 629 regulatory requirements, underscoring the intricacies of the regulatory landscape and the pressing need for effective compliance strategies.
Regulatory affairs in the pharmaceutical industry serve as a cornerstone for ensuring that drugs and medical devices are developed, tested, and marketed in compliance with established laws and regulations. This field not only safeguards public health but also facilitates the timely introduction of innovative therapies that can significantly enhance patient care. With an intricate landscape of compliance requirements, the expertise of regulatory professionals is essential for navigating the complexities of drug approval processes and maintaining the highest safety and quality standards.
Throughout this article, key insights have been highlighted, such as:
The discussion underscores how effective regulatory management not only accelerates the approval of new treatments but also reinforces public trust in the healthcare system. Furthermore, the role of organizations like bioaccess® exemplifies how strategic partnerships can streamline compliance processes and enhance market access, particularly for startups facing unique challenges in the regulatory landscape.
As the pharmaceutical sector continues to evolve, the significance of regulatory affairs will only grow. Leaders in the industry must prioritize the development of robust compliance strategies that adapt to changing regulations and technological advancements. By doing so, they can ensure that innovative therapies reach patients safely and efficiently, ultimately contributing to improved health outcomes and reinforcing the vital connection between regulatory compliance and public health.
What is the role of regulatory affairs in the pharmaceutical industry?
Regulatory affairs in pharma ensure compliance with laws and regulations governing the development, testing, manufacturing, and marketing of pharmaceutical products. This includes preparing and submitting compliance documents, communicating with regulatory agencies, and overseeing clinical trials.
What are some key responsibilities of compliance specialists in regulatory affairs?
Compliance specialists manage strategic planning for submissions, ensure adherence to evolving regulations, and facilitate communication among stakeholders to maintain compliance and product safety.
How do regulatory affairs professionals enhance compliance management?
They engage proactively with governing entities, leverage market insights, and utilize advanced tools like AI to streamline processes and enhance compliance.
Why is compliance management important in the pharmaceutical industry?
Compliance management is crucial for maintaining adherence to regulations and safeguarding public health, especially as the industry faces increasing compliance demands and complexities.
What challenges do medical device startups face in regulatory affairs?
Medical device startups encounter compliance hurdles, competitive pressures, recruitment challenges, and financial constraints when navigating regulatory processes.
How does bioaccess® assist medical device startups in Colombia?
Bioaccess® provides a comprehensive approach to advancing medical device studies, including site feasibility, investigator selection, regulatory compliance, project management, and reporting, helping to expedite clinical study approval.
What is the typical timeline for securing clinical study approval in Colombia compared to the US/EU?
Bioaccess® can expedite clinical study approval in Colombia to as little as 6-8 weeks, while the typical approval timeline in the US/EU is 6-12 months.
How does the expertise of regulatory affairs professionals benefit patients and healthcare systems?
Their expertise simplifies navigation through complex legal frameworks and accelerates the introduction of innovative therapies to the market, ultimately benefiting patients and healthcare systems.