


The primary objective of this article is to delineate the regulatory requirements for conducting medtech trials in Mexico, with a particular emphasis on the pivotal role of COFEPRIS in ensuring compliance and safety. This article substantiates its claims by outlining the regulatory framework, which encompasses:
These elements are highlighted as essential for facilitating the efficient conduct of clinical studies, all while prioritizing participant safety and product efficacy.
Navigating the intricate landscape of medical technology trials in Mexico necessitates a profound understanding of a robust regulatory framework, primarily governed by the Federal Commission for the Protection against Sanitary Risks (COFEPRIS). This article explores the essential regulatory requirements that shape the conduct of medtech trials, underscoring the opportunities for innovation and market access within a rapidly evolving sector.
However, as stakeholders strive for compliance, they frequently encounter significant challenges, including:
What strategies can be employed to effectively navigate these complexities and ensure successful trial outcomes?
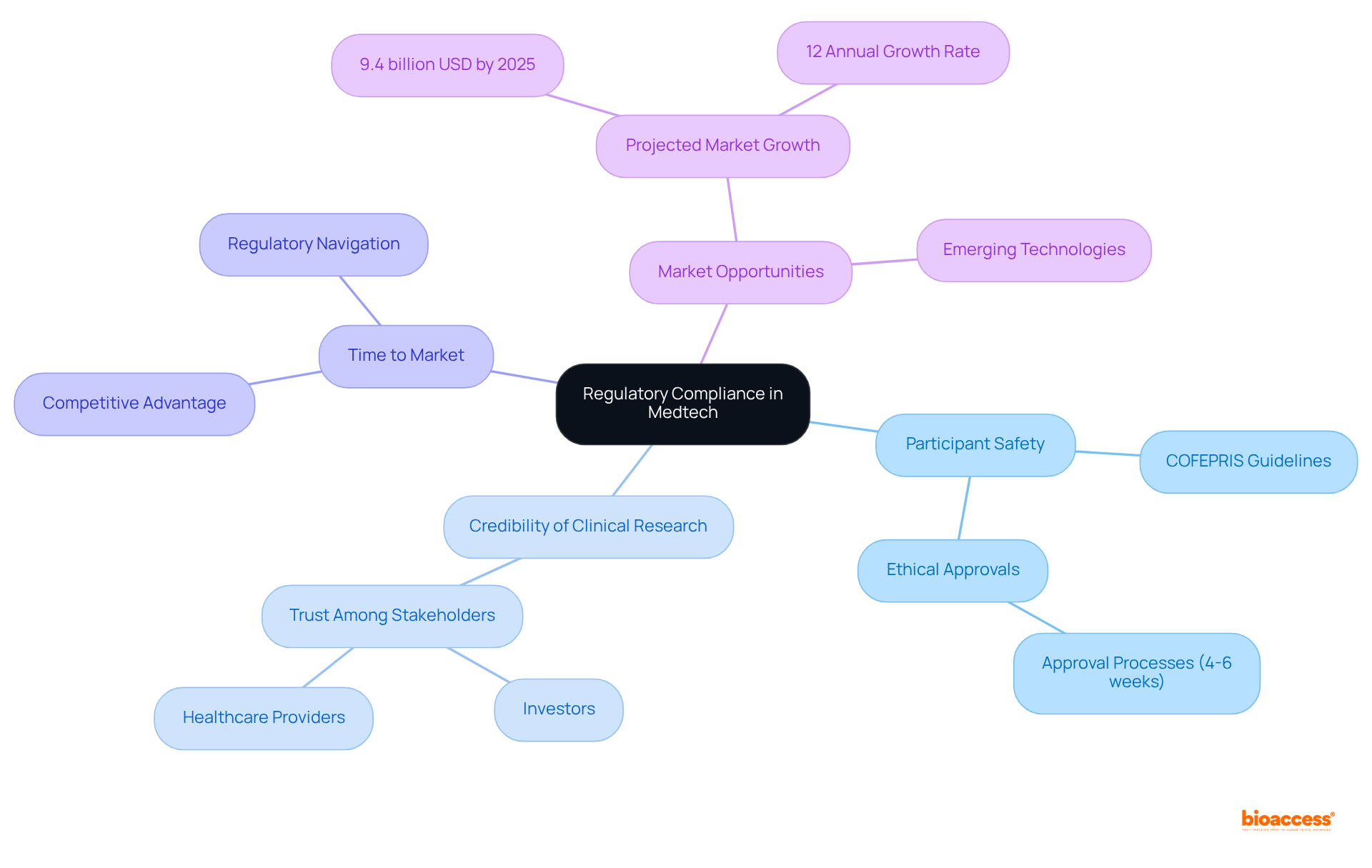
The oversight structure for Medtech trials in Mexico is primarily managed by the Federal Commission for the Protection against Sanitary Risks (COFEPRIS), which is essential for meeting the regulatory requirements for medtech trials in Mexico, ensuring the safety, efficacy, and quality of medical devices and clinical trials. This framework is anchored in the General Health Law and its complementary regulations, which outline the regulatory requirements for medtech trials in Mexico. Key components of this framework include:
Device Classification: Medical devices are categorized based on their risk levels, influencing the regulatory requirements they must meet. In line with the regulatory requirements for medtech trials in Mexico, most medical devices require pre-market authorization from COFEPRIS unless exempted, ensuring that products meet safety and efficacy standards before entering the market.
Ethical Clearances: Securing ethical clearances is essential prior to starting clinical studies, guaranteeing that research complies with ethical standards. Notably, bioaccess® enables this process, providing ethical consent in 4-6 weeks. Adhering to regulatory requirements for medtech trials in Mexico, particularly Good Clinical Practice (GCP) guidelines, is critical for maintaining the integrity of clinical trials and protecting participant rights.
Simplified Authorization System: Recent updates have introduced a simplified authorization system for Class I and II products, which has decreased average processing times, facilitating manufacturers in bringing their products to market.
COFEPRIS Authorization Process: The authorization process consists of six steps, including determining device classification, appointing a Registration Holder in the country, demonstrating an audited quality system, preparing a registration dossier, paying application fees, and obtaining a registration certificate upon positive review.
Recent updates to the General Health Law in the country are anticipated to enhance the oversight landscape, particularly with the introduction of new Good Manufacturing Practices (GMP) guidelines, which aim to streamline approval processes for high-risk products and potentially reduce approval times by up to 30%. The medical devices market in the country is projected to reach 8 billion USD from 2017 to 2029. Understanding this governance structure is essential for participants aiming to navigate the regulatory requirements for medtech trials in Mexico while conducting clinical studies efficiently. With COFEPRIS's commitment to improving its registration procedure, the environment for Medtech studies is becoming increasingly favorable, enabling quicker market access for innovative medical technologies.
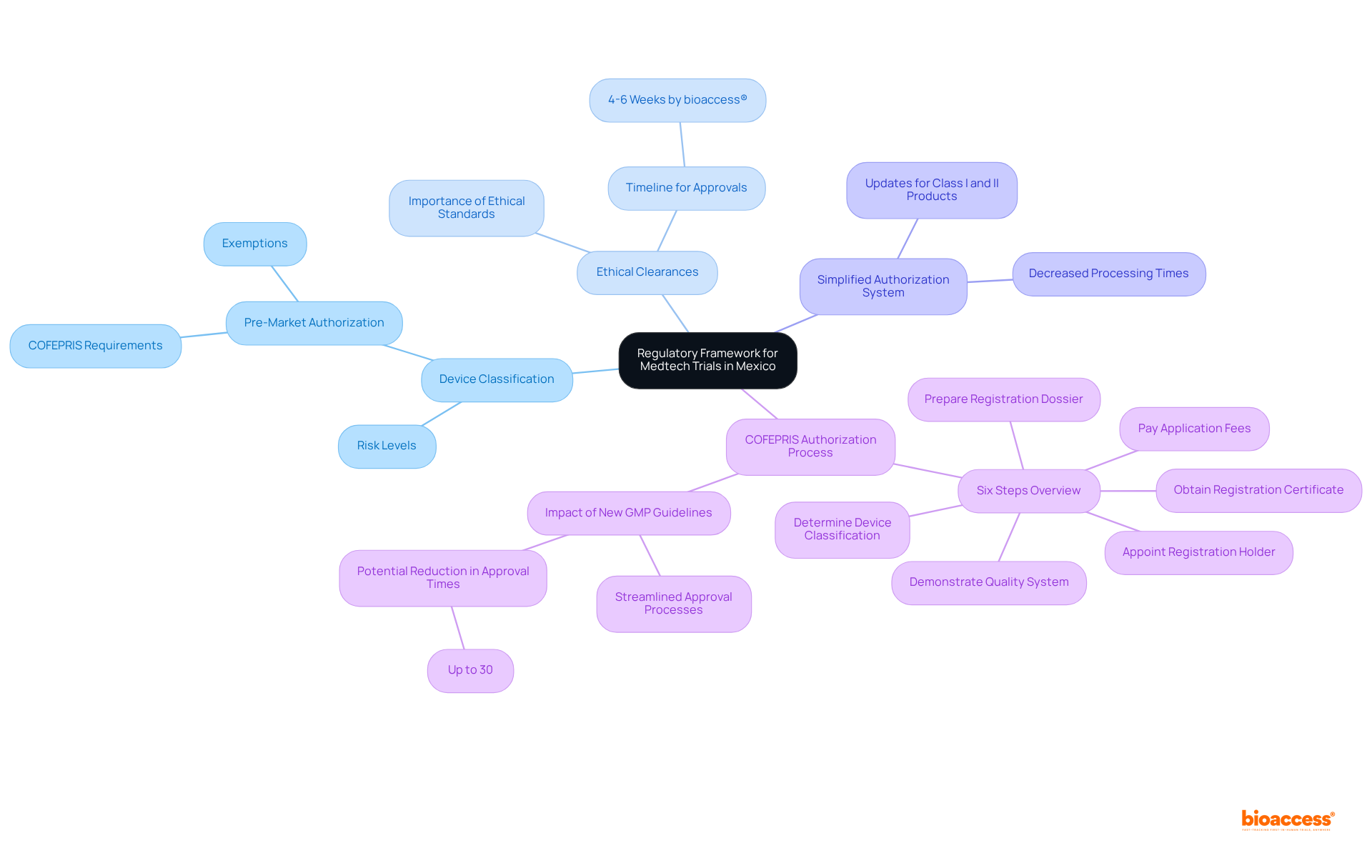
Conducting Medtech studies in Mexico necessitates strict compliance with the regulatory requirements for medtech trials in Mexico. It is essential that all clinical research protocols are submitted to the Federal Commission for Protection Against Sanitary Risk (COFEPRIS) for authorization. This process necessitates comprehensive details about the study design, objectives, and methodology. Documentation must be provided in Spanish, and while COFEPRIS aims for a review duration of 30 working days, the typical authorization time can extend to approximately 10 months due to preliminary evaluations.
Additionally, researchers must secure ethical approval from an Institutional Review Board (IRB) in accordance with the regulatory requirements for medtech trials in Mexico before commencing experiments. Compliance with Good Clinical Practice (GCP) guidelines is vital, ensuring that trials are conducted ethically and prioritizing participant safety. These governing frameworks are crucial for maintaining the integrity of clinical research and safeguarding the rights of participants.
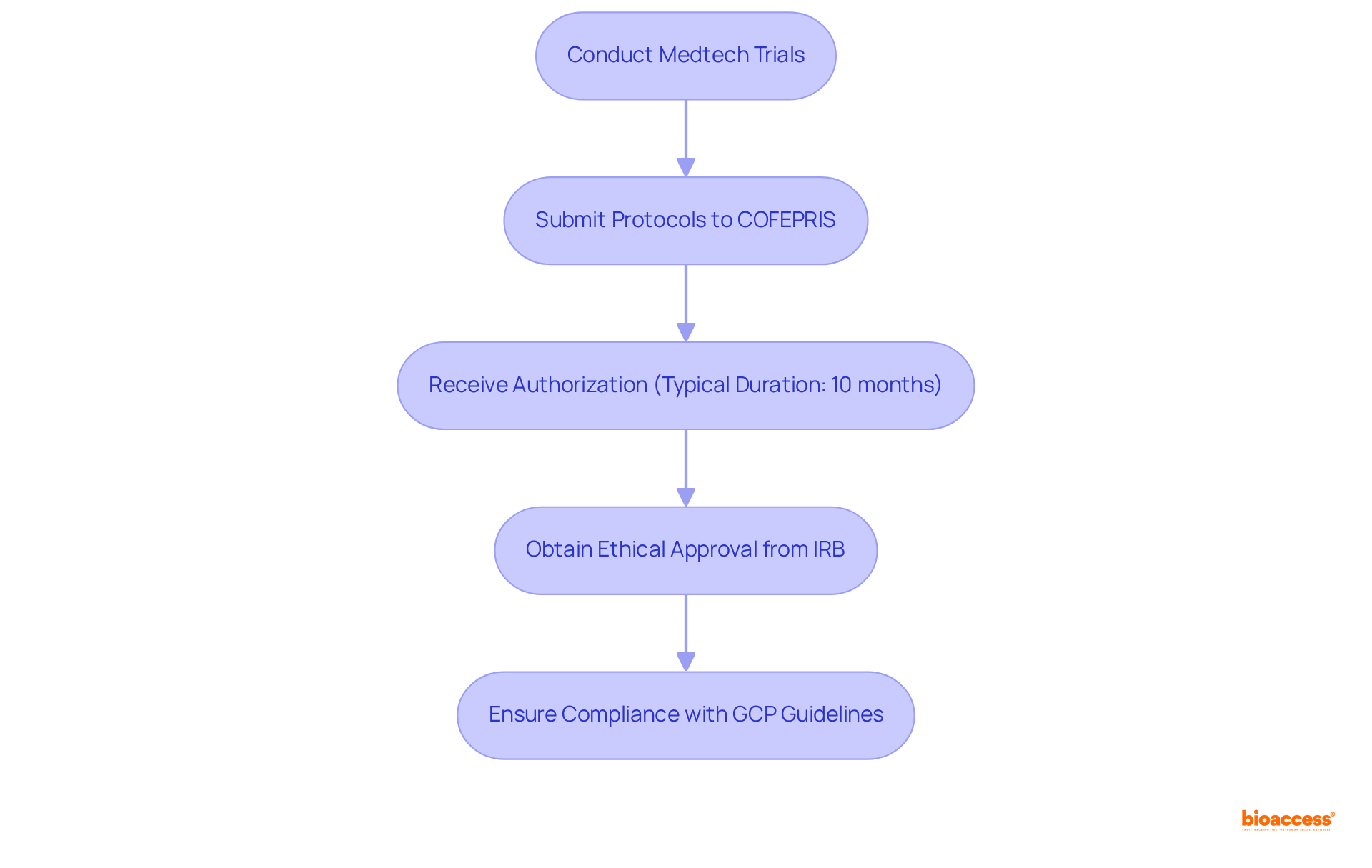
Navigating the regulatory requirements for medtech trials in Mexico presents several significant challenges. The complexity of the regulatory requirements for medtech trials in Mexico often leads to delays, particularly when submissions are incomplete or misclassified.
Although ethical clearances in Mexico can be obtained in 4-6 weeks, the submission process to COFEPRIS can still be lengthy, with processing times stretching for months, leading to backlogs that obstruct prompt market entry. Furthermore, the necessity for local trials for specific products complicates the validation process, particularly for international firms unacquainted with the regulatory requirements for medtech trials in Mexico.
Language barriers exacerbate these challenges, as all documentation must be submitted in Spanish, necessitating professional translators specializing in medical terminology to ensure accuracy and compliance. The evolving nature of regulations, particularly with COFEPRIS's ongoing efforts to simplify and expedite the registration and approval of medical devices over the past three years, adds another layer of complexity.
Stakeholders must remain vigilant and adaptable to successfully navigate these hurdles and ensure compliance, as missteps can lead to significant delays and increased costs.
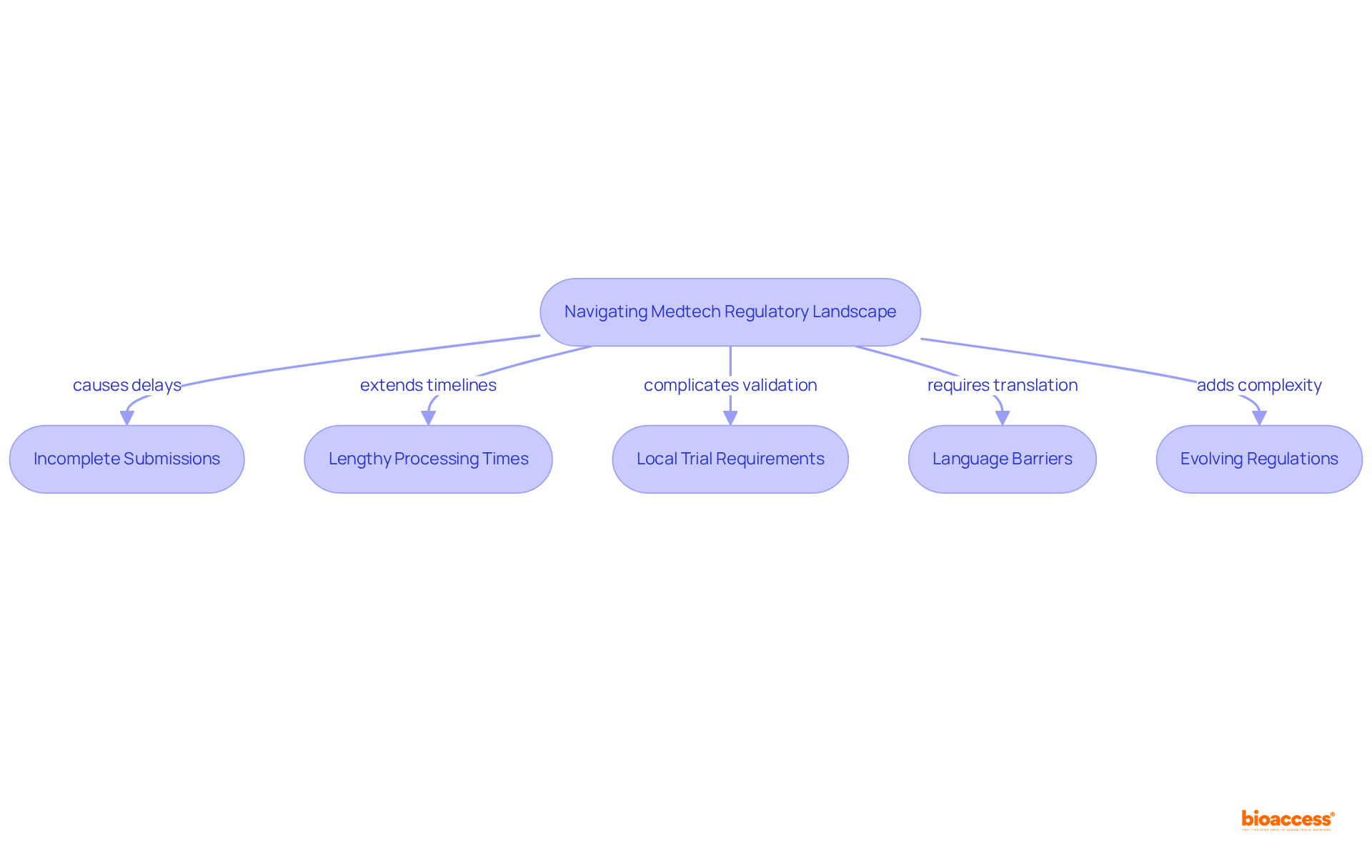
The regulatory requirements for medtech trials in Mexico play a pivotal role in shaping Medtech innovations in the country. Adhering to the regulatory requirements for medtech trials in Mexico not only safeguards participant safety but also bolsters the credibility of clinical research. Businesses that skillfully maneuver through the compliance environment can greatly shorten their time to market, obtaining a competitive advantage in the rapidly evolving Medtech industry.
For instance, organizations that adhere to the guidelines established by COFEPRIS, the governing body in the country, frequently undergo accelerated approval processes, with ethical approvals attainable in as few as 4-6 weeks. This efficiency is vital, particularly as the medical technology market in the country is expected to hit 9.4 billion USD by 2025, with the current revenue of the medical devices sector estimated at 8 billion USD, underscoring the urgency for companies to seize emerging opportunities.
Furthermore, compliance fosters trust among stakeholders, including investors and healthcare providers, which is essential for the successful commercialization of new products. As the governing environment changes, ensuring adherence to the regulatory requirements for medtech trials in Mexico will be essential for companies aiming to capitalize on the expanding healthcare market in the country, where the medical device sector is increasing at an average annual rate of 12%, exceeding the global average.
As noted by Ana Criado, Director of Regulatory Affairs, "Understanding the classification and compliance landscape is vital for manufacturers aiming to navigate the complexities of the Mexican market effectively."
In summary, the implications of regulatory compliance extend beyond mere adherence; they are integral to the strategic positioning and success of Medtech innovations in Mexico.
Understanding the regulatory landscape for Medtech trials in Mexico is crucial for ensuring the safety and efficacy of medical devices. This framework, primarily overseen by COFEPRIS, encompasses vital components such as device classification, ethical clearances, and a simplified authorization system. By navigating these regulatory requirements effectively, stakeholders can facilitate timely market access for innovative medical technologies while adhering to necessary ethical and safety standards.
Key insights from the article highlight the importance of:
Despite the challenges posed by lengthy authorization times and language barriers, recent updates to the General Health Law promise to improve the regulatory environment, making it more favorable for Medtech studies.
In light of the projected growth of the medical devices market in Mexico, stakeholders are encouraged to remain informed and adaptable in their approach to regulatory compliance. By doing so, they can not only safeguard participant rights but also position their innovations for success in a rapidly evolving industry. Embracing these regulatory requirements is not merely a legal obligation; it is a strategic advantage that can lead to accelerated market entry and enhanced credibility within the healthcare sector.
What is the primary regulatory body overseeing Medtech trials in Mexico?
The primary regulatory body overseeing Medtech trials in Mexico is the Federal Commission for the Protection against Sanitary Risks (COFEPRIS).
What laws govern the regulatory framework for Medtech trials in Mexico?
The regulatory framework for Medtech trials in Mexico is anchored in the General Health Law and its complementary regulations.
How are medical devices classified in Mexico?
Medical devices in Mexico are categorized based on their risk levels, which influences the regulatory requirements they must meet.
Is pre-market authorization required for most medical devices in Mexico?
Yes, most medical devices require pre-market authorization from COFEPRIS unless exempted, ensuring products meet safety and efficacy standards before entering the market.
What is the importance of ethical clearances in Medtech trials?
Securing ethical clearances is essential prior to starting clinical studies to ensure compliance with ethical standards and protect participant rights.
How long does bioaccess® take to provide ethical consent?
Bioaccess® enables the ethical consent process to be completed in 4-6 weeks.
What recent updates have been made to the authorization system for medical devices?
Recent updates have introduced a simplified authorization system for Class I and II products, which has decreased average processing times for manufacturers.
What are the steps involved in the COFEPRIS authorization process?
The COFEPRIS authorization process consists of six steps: determining device classification, appointing a Registration Holder in the country, demonstrating an audited quality system, preparing a registration dossier, paying application fees, and obtaining a registration certificate upon positive review.
What changes are expected from the recent updates to the General Health Law?
Recent updates are anticipated to enhance the oversight landscape and introduce new Good Manufacturing Practices (GMP) guidelines, which aim to streamline approval processes for high-risk products and potentially reduce approval times by up to 30%.
What is the projected growth of the medical devices market in Mexico?
The medical devices market in Mexico is projected to reach 8 billion USD from 2017 to 2029.
Why is it important to understand the governance structure for Medtech trials in Mexico?
Understanding the governance structure is essential for participants aiming to navigate the regulatory requirements efficiently while conducting clinical studies in Mexico.