


The pursuit of scientific knowledge relies heavily on the reliability of research findings, which form the foundation for informed decision-making and effective practices across various fields, particularly in clinical settings. Understanding reliability in science not only bolsters the credibility of results but also enables researchers to generalize their findings confidently to broader populations.
However, how can researchers guarantee that their measurements are both consistent and valid, especially when patient care and treatment outcomes are at stake? This article explores the definition of reliability in scientific research, its critical importance, the different types of reliability, and the intricate relationship between reliability and validity, illuminating the path toward more trustworthy and impactful scientific inquiry.
The reliability in science definition is fundamentally characterized by the consistency and stability of data or findings obtained from a study. It reflects the extent to which an instrument or method yields the same outcomes under consistent conditions. For instance, when a researcher measures the same variable multiple times and consistently obtains comparable findings, that measurement is deemed reliable. This concept is crucial in research as it strengthens the reliability in science definition, which bolsters the credibility of findings and underpins the validity of conclusions drawn from the data.
Dependability can be assessed through various methods, including test-retest measures, which evaluate the consistency of outcomes when the same assessment is administered to the same subjects over time. In clinical studies, achieving high test-retest consistency is vital; benchmarks for acceptable consistency coefficients, such as an intraclass correlation coefficient (ICC) value above 0.70, underscore the significance of dependability in medical assessments. Furthermore, inter-rater consistency, which assesses the agreement among various observers, is essential in studies involving subjective evaluations, ensuring that variability in diagnoses is minimized. For example, employing standardized rating scales can significantly reduce variability in clinical evaluations.
Moreover, practical applications of dependability in clinical environments can be illustrated by the mean of three blood pressure readings, which enhances reliability. In general, the reliability in science definition serves as a cornerstone of scientific measurements, as it strengthens the robustness of findings and supports informed decision-making in clinical practice. The principles of data integrity, encapsulated in the acronym ALCOA—Attributable, Legible, Contemporaneous, Original, and Accurate—provide a clear framework for understanding the standards that underpin dependable studies.

Dependability in studies is of utmost importance. The reliability in science definition shows that reliable findings empower researchers to confidently extend results to larger populations, significantly enhancing the relevance of outcomes for medical practice. High consistency indicates that findings are stable and trustworthy, which is essential for establishing reliability in science definition and confidence in the research process. In clinical settings, for instance, reliable measurements can lead to improved patient outcomes by ensuring that treatments are based on consistent and accurate data.
Moreover, the reliability in science definition is vital for the replication of studies, which is a cornerstone of scientific validation. It allows other researchers to verify results and build upon previous work. At bioaccess®, our comprehensive clinical trial management services—including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting—are meticulously designed to ensure that the studies we conduct adhere to the reliability in science definition and meet the highest standards of trustworthiness.
By leveraging our expertise in managing various types of studies, such as Early-Feasibility and First-In-Human studies, we contribute to generating dependable data that illustrates the reliability in science definition, significantly impacting patient care and advancing medical knowledge. Are you ready to enhance the reliability of your clinical research? Let’s collaborate to ensure your studies achieve the highest standards of excellence.
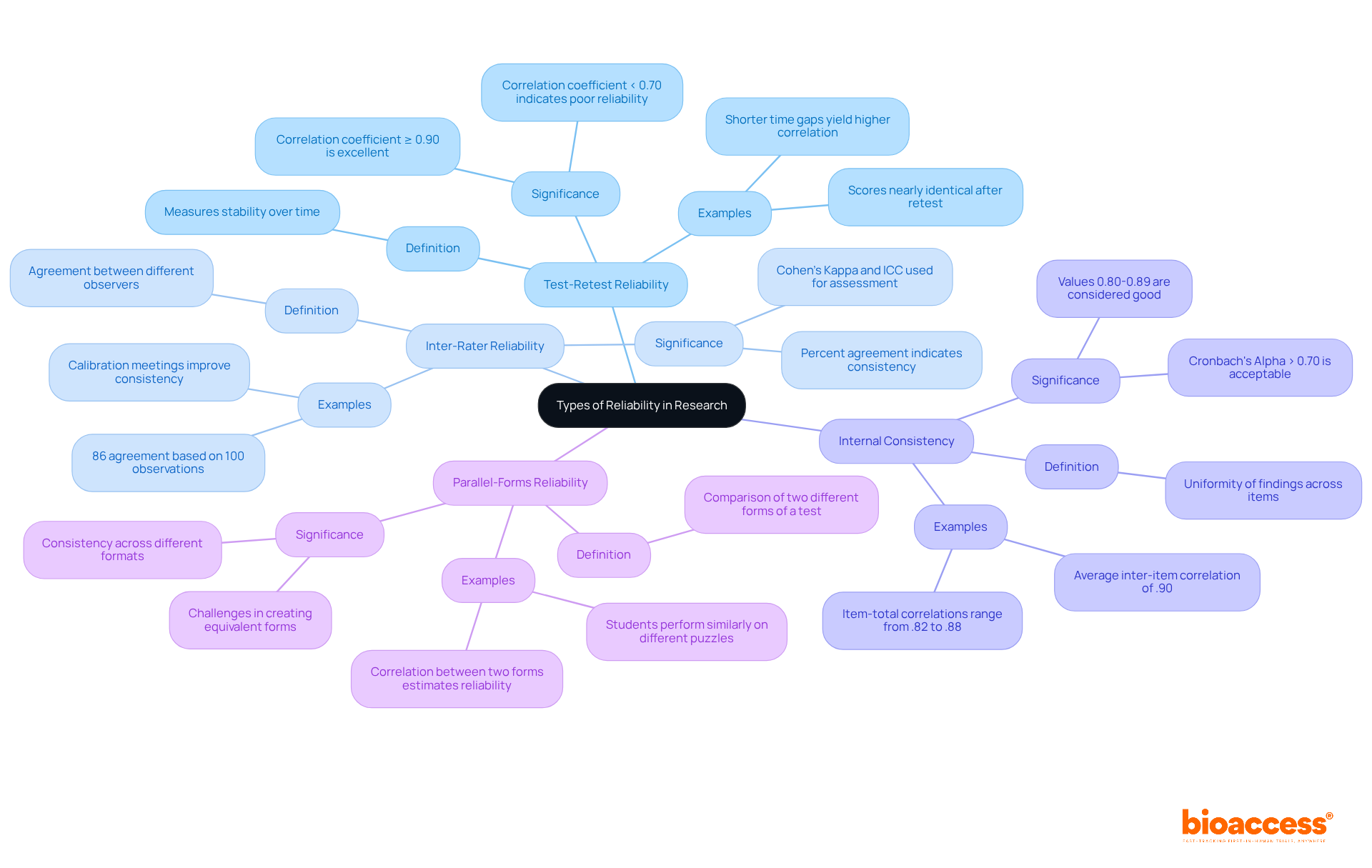
In the realm of clinical research, dependability is paramount. Various forms of dependability serve unique purposes in evaluating measurements, which ties into the reliability in science definition, ensuring that study results are credible and reliable. The main types include:
Test-Retest Reliability: This measures the stability of results over time by administering the same test to the same subjects at different points. A high test-retest consistency score is indicated by a correlation coefficient of 0.90 or higher, while a coefficient below 0.70 may suggest poor consistency, providing a spectrum of assessment.
Inter-Rater Reliability: This assesses the degree of agreement between different observers or raters measuring the same phenomenon. High inter-rater consistency is evident when several evaluators provide similar ratings for the same patient assessments. Methods such as Cohen’s Kappa and Intraclass Correlation Coefficient (ICC) are frequently employed to assess inter-rater consistency, ensuring trustworthy measurements across different evaluators. For instance, a percent agreement of 86% between two raters based on 100 observations exemplifies strong inter-rater reliability.
Internal Consistency: This evaluates the uniformity of findings across items within a single assessment, ensuring that all components measure the same construct. A Cronbach's Alpha value above 0.70 is generally acceptable, while values of 0.80-0.89 are considered good, and values below 0.70 indicate a need for improvement.
Parallel-Forms Reliability: This involves comparing the results of two different forms of the same test to determine if they yield similar results. This method is particularly beneficial when evaluating the dependability of instruments designed to measure the same construct through various formats. However, producing two comparable forms can be challenging, complicating the evaluation of accuracy.
Every form of dependability, as described in the reliability in science definition, is essential for guaranteeing that study results are credible and can be relied upon, ultimately enhancing the quality of clinical investigations and patient treatment.
The interaction between dependability and validity stands as a cornerstone of research methodology. The reliability in science definition refers to the consistency of a measurement, while validity assesses the accuracy of what that measurement is intended to evaluate. For instance, consider a scale that consistently provides the same incorrect weight; this exemplifies reliability without validity. In contrast, a valid measure, such as an intelligence test, must produce consistent results across various administrations to be deemed valid. This relationship is especially critical in clinical studies, where the implications of precision can directly influence patient outcomes.
Ongoing studies underscore that although a measurement may be dependable, it does not guarantee validity. Grasping this difference is crucial for investigators striving to generate credible and significant results, as it relates to the reliability in science definition. Expert opinions emphasize that both the reliability in science definition and validity are essential for ensuring that findings are trustworthy and meaningful, ultimately guiding effective decision-making in clinical practice. Furthermore, internal validity, defined as the trustworthiness of conclusions regarding causal relationships, plays a vital role in establishing the credibility of research findings. As Campbell & Stanley noted, understanding these concepts is crucial for researchers to draw valid inferences and make informed decisions based on their studies.

Reliability in scientific research stands as a cornerstone concept, emphasizing the necessity of consistency and stability in data collection and analysis. It forms the foundation upon which credible findings are constructed, empowering researchers to confidently generalize results and make informed decisions across various fields, especially in clinical settings. A solid grasp of reliability is crucial for ensuring that research outcomes are trustworthy, ultimately contributing to advancements in medical practice and knowledge.
Key points throughout this discussion underscore the different types of reliability, such as:
Each type is vital in ensuring that measurements are dependable and replicable, which is essential for validating scientific findings. The interplay between reliability and validity further highlights that while a measurement may be consistent, it must also accurately reflect its intended purpose to be considered truly effective.
The importance of reliability in research cannot be overstated. It not only elevates the quality of scientific investigations but also has a direct impact on patient care and treatment outcomes. Researchers must prioritize reliability in their studies, employing rigorous methods to ensure that their findings are both credible and applicable. By doing so, they contribute to a body of knowledge that supports informed decision-making and fosters trust in scientific inquiry.
What is the definition of reliability in scientific research?
Reliability in scientific research refers to the consistency and stability of data or findings obtained from a study. It indicates the extent to which an instrument or method produces the same outcomes under consistent conditions.
Why is reliability important in research?
Reliability is crucial in research as it enhances the credibility of findings and supports the validity of conclusions drawn from the data.
How can dependability be assessed in scientific studies?
Dependability can be assessed through various methods, including test-retest measures, which evaluate the consistency of outcomes when the same assessment is administered to the same subjects over time.
What is the significance of test-retest consistency in clinical studies?
High test-retest consistency is vital in clinical studies, with acceptable consistency coefficients, such as an intraclass correlation coefficient (ICC) value above 0.70, indicating significant dependability in medical assessments.
What is inter-rater consistency and why is it important?
Inter-rater consistency assesses the agreement among various observers in studies involving subjective evaluations. It is important because it minimizes variability in diagnoses.
Can you provide an example of how to enhance reliability in clinical environments?
An example of enhancing reliability in clinical environments is taking the mean of three blood pressure readings, which improves the reliability of the measurement.
What framework supports the principles of data integrity in reliable studies?
The principles of data integrity are encapsulated in the acronym ALCOA—Attributable, Legible, Contemporaneous, Original, and Accurate—which provides a clear framework for understanding the standards that underpin dependable studies.