


This article highlights the critical role of reliability science in the medical field, underscoring its importance in ensuring that medical devices and pharmaceuticals consistently fulfill their intended functions. By doing so, it significantly enhances patient safety and clinical outcomes. The discussion delves into the evolution of reliability metrics, the necessity of rigorous testing, and real-world applications that illustrate how these principles effectively mitigate risks and elevate healthcare quality.
In the ever-evolving Medtech landscape, understanding reliability science is paramount. It addresses key challenges faced by the industry, paving the way for innovations that prioritize patient well-being. As we explore these concepts, consider how they relate to your own experiences in clinical research.
Ultimately, collaboration among stakeholders is essential for advancing reliability science. By working together, we can ensure that the principles of reliability are not just theoretical but are actively applied to improve healthcare outcomes. The next steps involve fostering partnerships that drive these initiatives forward.
Understanding the complexities of reliability science is crucial for navigating the multifaceted landscape of healthcare and clinical research. This field not only assesses the dependability of medical devices and pharmaceuticals but also significantly contributes to enhancing patient safety and improving healthcare outcomes. As the demand for high-quality, reliable medical technologies continues to rise, stakeholders must consider: how can they effectively integrate reliability principles into their practices to tackle the challenges they encounter?
The Medtech landscape is evolving rapidly, and with it comes the need for robust reliability frameworks. By addressing key challenges, such as device failures and inconsistent pharmaceutical efficacy, organizations can foster a culture of safety and trust. This is where the role of bioaccess becomes pivotal, offering innovative solutions that align with reliability science to ensure optimal patient outcomes.
Collaboration among stakeholders is essential in this endeavor. By sharing insights and best practices, the healthcare community can collectively enhance the reliability of medical technologies. The next steps involve not only adopting these principles but also actively engaging in discussions that drive the industry forward.
The reliability science meaning is important in reliability research, which plays a crucial role in clinical studies by investigating how systems or components consistently perform their intended functions under specified conditions over a defined period. This field employs various methodologies and metrics to evaluate the dependability of products, processes, and systems, which is essential for ensuring that medical instruments and pharmaceuticals meet safety and effectiveness standards before entering the market. The reliability science meaning is underscored by the significance of reliability research in its ability to mitigate risks, bolster patient safety, and enhance overall healthcare outcomes.
For instance, high trust organizations (HROs) prioritize predictable behaviors and consistent processes, which are vital for achieving excellence in patient care. By integrating reliability science meaning into medical device development, organizations not only enhance adherence to regulatory requirements but also foster a culture of safety that ultimately leads to improved patient experiences. Statistics indicate that healthcare systems adopting dependable principles can significantly reduce variability in clinical care delivery, thereby enhancing outcomes and decreasing costs.
Expert opinions emphasize that attaining high dependability is an ongoing journey. It requires a commitment to learning and adjusting practices to ensure patient safety and high-quality care. As you consider your own challenges in clinical research, reflect on how incorporating the reliability science meaning could transform your approach and outcomes.
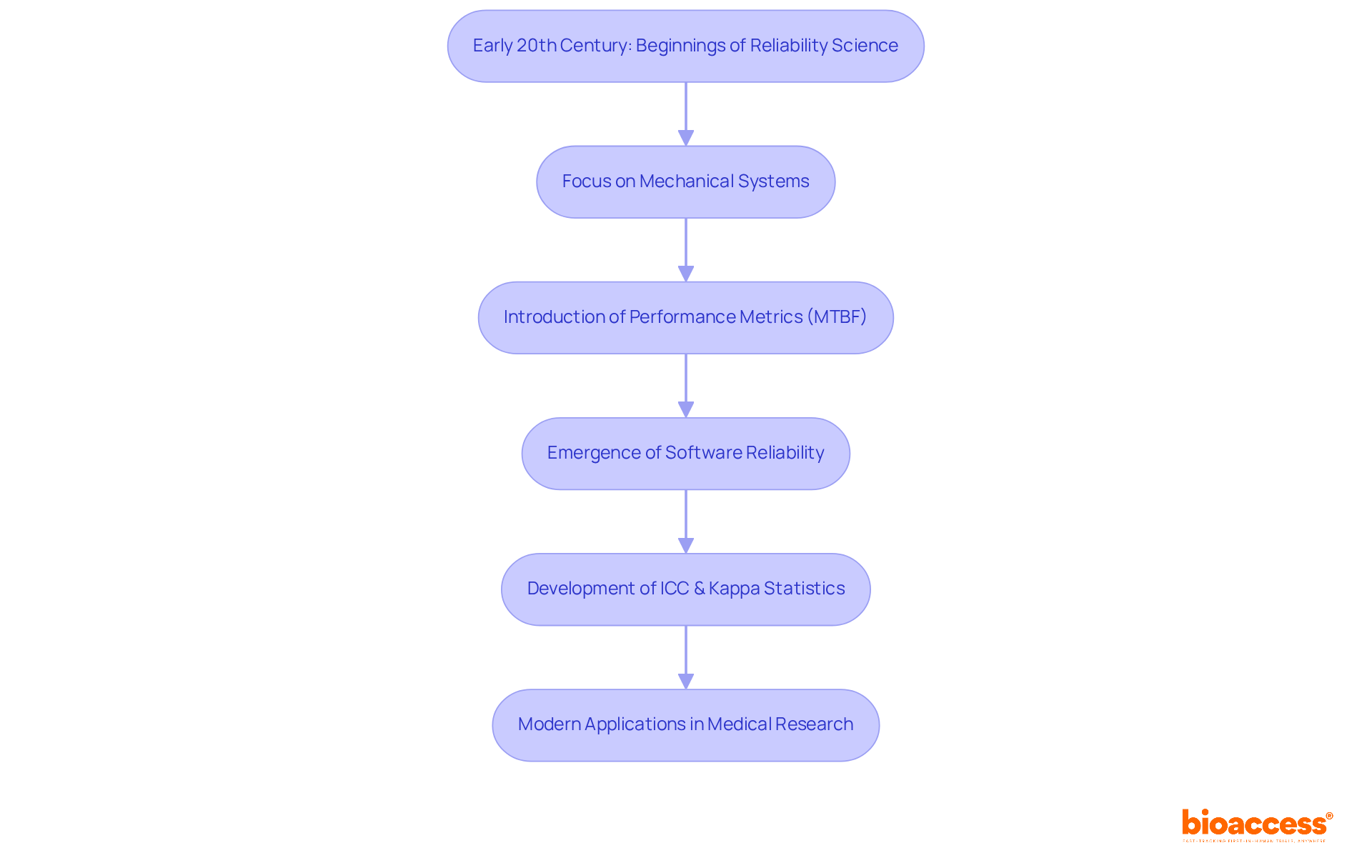
The development of dependable methods is closely related to reliability science meaning, tracing its roots back to the early 20th century, primarily emerging from engineering and statistical fields. Initially centered on mechanical systems, this domain has expanded to include software and complex systems, mirroring technological advancements. Notable milestones include the establishment of performance metrics like Mean Time Between Failures (MTBF) and the formulation of testing standards by organizations such as the American National Standards Institute (ANSI).
In the realm of medical research, the reliability science meaning has become increasingly vital, particularly concerning regulatory compliance and patient safety. This evolution has spurred the development of more robust approaches in trial design and execution, ultimately enhancing the reliability of outcomes. The introduction of the Intraclass Correlation Coefficient (ICC) and Kappa statistics has provided essential tools for assessing inter-rater consistency, ensuring uniformity across evaluations.
Effective trial management services—encompassing:
are crucial for leveraging these trust metrics. As the field progresses, understanding reliability science meaning through the application of dependability metrics in research trials remains indispensable for achieving high-quality study results. This commitment not only bolsters local economies through job creation and economic development but also enhances healthcare and fosters international collaboration.
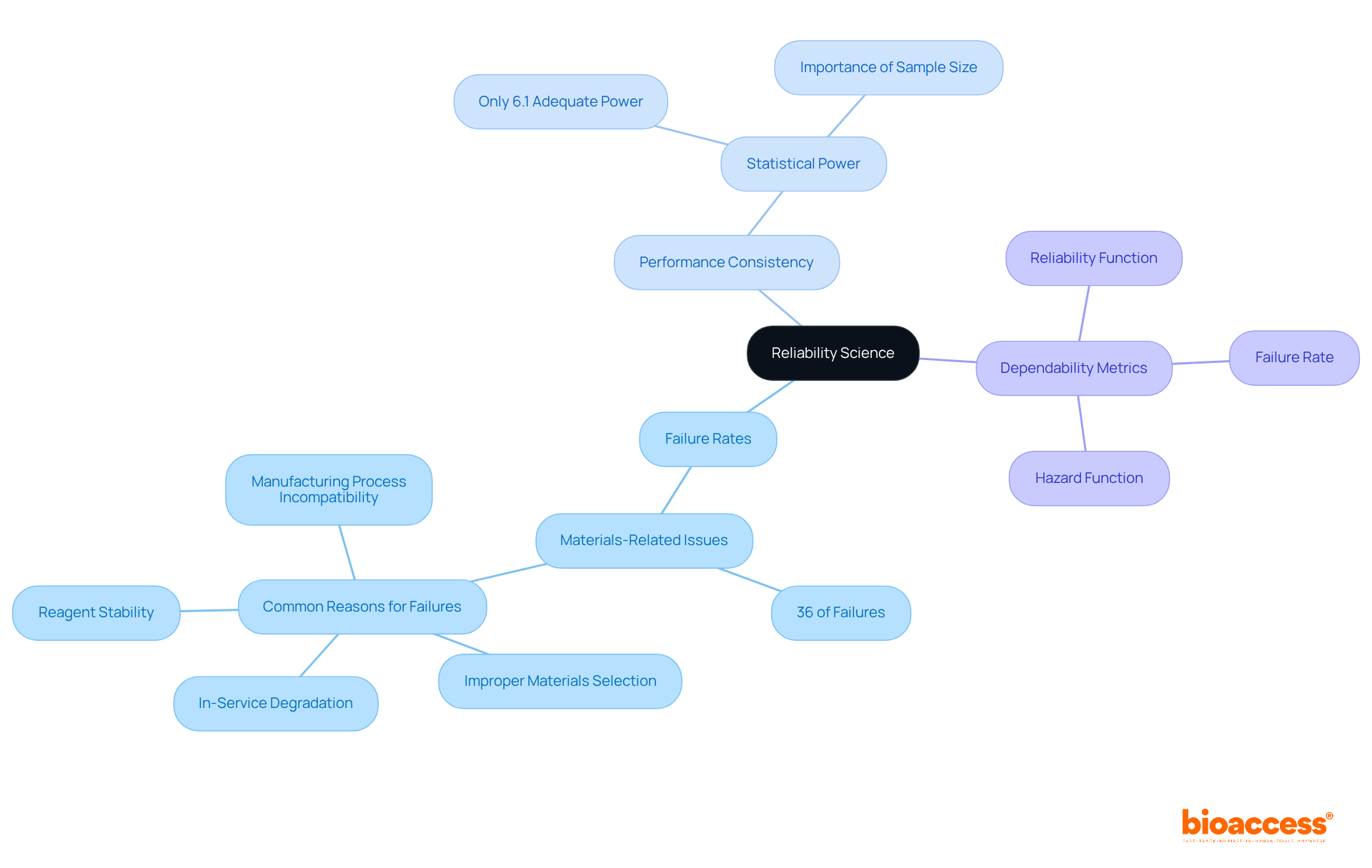
The reliability science meaning plays a crucial role in clinical research, fundamentally defined by the evaluation of failure rates, performance consistency, and the establishment of dependability metrics. Key metrics in this field, such as the Reliability Function, Hazard Function, and Failure Rate, illustrate the reliability science meaning by quantifying the likelihood of a product or system performing successfully over time. In the Medtech landscape, the significance of consistency is underscored by stringent testing and validation procedures that ensure medical tools and therapies are both effective and safe for patient use.
Statistics reveal that materials-related problems account for 36% of medical instrument failures, highlighting the critical need for comprehensive failure rate evaluations during equipment testing. Furthermore, only 6.1% of research published in British orthopedic journals demonstrated sufficient statistical power, which emphasizes the necessity for robust validity metrics to enhance performance consistency in clinical trials. These insights are vital for researchers and developers aiming to meet regulatory standards and improve product quality, ultimately leading to better patient outcomes.
Understanding these components is essential for navigating the complexities of clinical research, which relates to the reliability science meaning. By prioritizing dependability metrics and addressing the challenges within the Medtech sector, stakeholders can foster collaboration and drive innovation. The next steps involve a commitment to rigorous evaluation processes and a focus on enhancing the reliability of medical technologies.
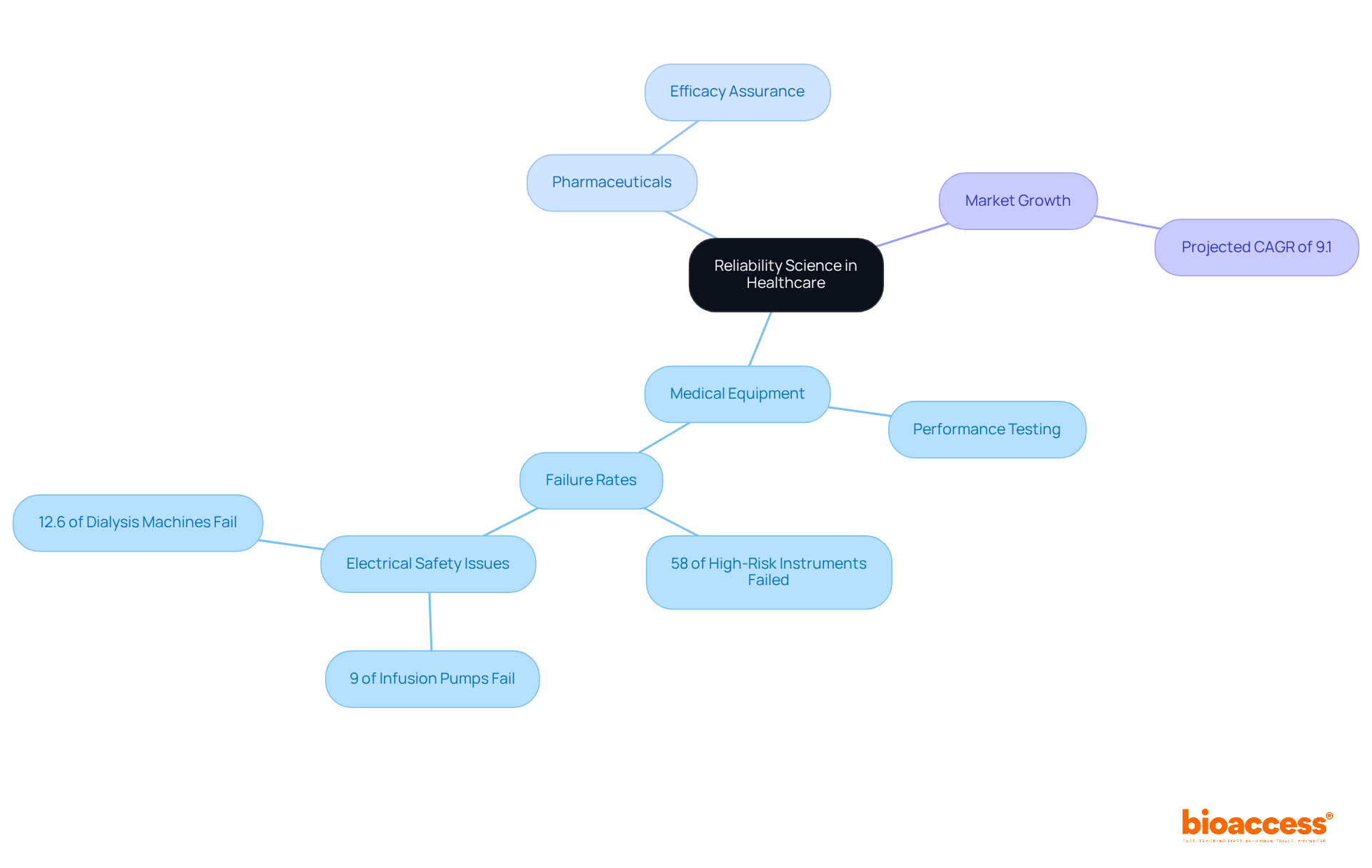
Reliability research is vital in healthcare, particularly in the development and management of medical equipment and pharmaceuticals. Performance testing is crucial for devices like pacemakers, which must operate flawlessly over extended periods to minimize the risk of malfunction. In the pharmaceutical sector, assurance science guarantees that drug formulations retain their efficacy throughout their shelf life, thereby protecting patient health. A systematic review of studies on medical instrument dependability found that around 58% of high-risk instruments failed performance tests, underscoring the necessity for stringent dependability evaluations.
Moreover, the market for medical device maintenance is projected to grow at a compound annual growth rate (CAGR) of 9.1% from 2021 to 2026, reflecting an increasing emphasis on reliability in healthcare. Companies such as bioaccess® utilize dependable methodologies to enhance clinical trial processes, ensuring that studies are conducted effectively and that products meet rigorous safety and efficacy standards before reaching the market. These applications highlight the critical role of reliability science in advancing medical technology and improving patient outcomes.
The essence of reliability science is foundational in ensuring that systems, particularly in healthcare, perform consistently and safely over time. Understanding reliability science allows stakeholders to appreciate its critical role in enhancing patient care and safety through rigorous evaluation and adherence to established metrics.
Throughout this discussion, we’ve explored key concepts such as the historical evolution of reliability science, its core characteristics, and practical applications. Methodologies like performance testing and dependability metrics significantly contribute to the overall reliability of medical devices and pharmaceuticals, ultimately leading to improved healthcare outcomes. Moreover, the significance of high-trust organizations in fostering a culture of safety and consistency underscores the importance of integrating reliability science into clinical practices.
In light of these insights, embracing the principles of reliability science is not merely an option but a necessity for advancing healthcare quality. As the landscape of medical technology continues to evolve, a commitment to reliability will drive innovation, enhance safety, and ultimately protect patient health. Stakeholders in the healthcare sector are encouraged to prioritize reliability science in their strategies and practices, ensuring that patient safety remains at the forefront of their endeavors.
What is the definition of reliability science?
Reliability science is the study of how systems or components consistently perform their intended functions under specified conditions over a defined period. It employs various methodologies and metrics to evaluate the dependability of products, processes, and systems.
Why is reliability science important in clinical studies?
Reliability science is crucial in clinical studies as it ensures that medical instruments and pharmaceuticals meet safety and effectiveness standards before entering the market. It helps mitigate risks, bolster patient safety, and enhance overall healthcare outcomes.
How do high trust organizations (HROs) relate to reliability science?
High trust organizations prioritize predictable behaviors and consistent processes, which are essential for achieving excellence in patient care. By integrating reliability science into their practices, they enhance adherence to regulatory requirements and foster a culture of safety.
What impact does reliability science have on healthcare systems?
Healthcare systems that adopt dependable principles can significantly reduce variability in clinical care delivery, leading to improved outcomes and decreased costs.
What is necessary for achieving high dependability in healthcare?
Achieving high dependability is an ongoing journey that requires a commitment to learning and adjusting practices to ensure patient safety and high-quality care.
How can incorporating reliability science transform clinical research outcomes?
Incorporating the meaning of reliability science into clinical research can lead to improved approaches and outcomes by emphasizing the importance of consistent performance and safety in healthcare practices.