


In the rapidly evolving landscape of clinical research, trial feasibility reports in Bulgaria emerge as a cornerstone for successful study execution. These reports assess site capabilities and patient demographics while ensuring compliance with stringent regulatory requirements. This paves the way for informed decision-making by sponsors and researchers alike. As Bulgaria aligns itself with European Union regulations, navigating the increasingly complex requirements for these assessments presents a significant challenge.
What are the essential components of trial feasibility reports, and how do they impact the efficiency and success of clinical research in this pivotal European hub? Understanding these elements is crucial for stakeholders aiming to optimize their research strategies.
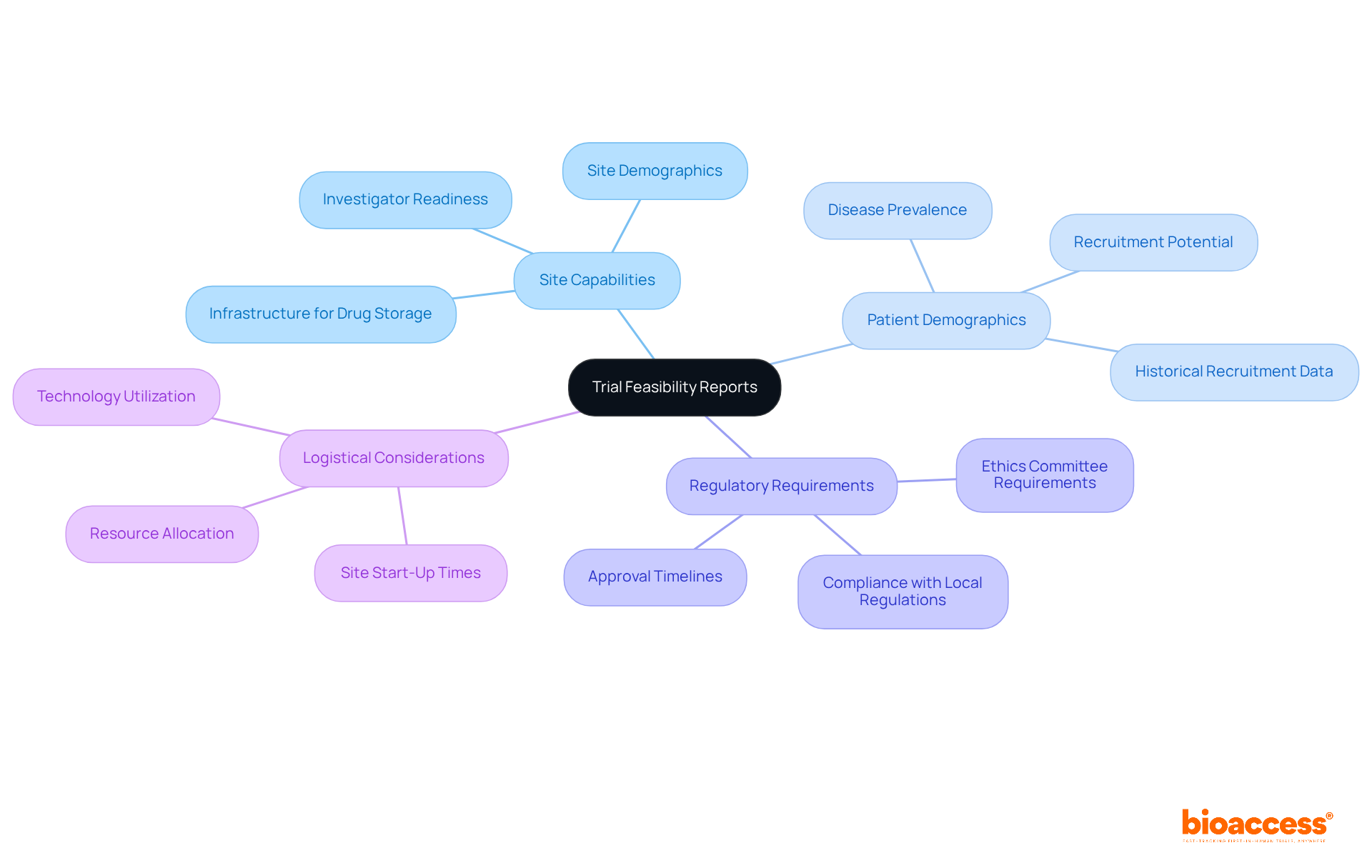
Feasibility assessments are crucial documents that evaluate the practicality of conducting a study at a specific location or within a defined area. These assessments meticulously analyze various factors, including:
In Bulgaria, where the landscape for medical research is rapidly evolving, the requirements for trial feasibility reports in Bulgaria are essential to ensure that studies are conducted effectively and ethically. They serve as a vital resource for sponsors and researchers, enabling informed decisions regarding study design and execution. By addressing potential challenges and adhering to local practices, feasibility assessments significantly enhance the likelihood of successful medical research.
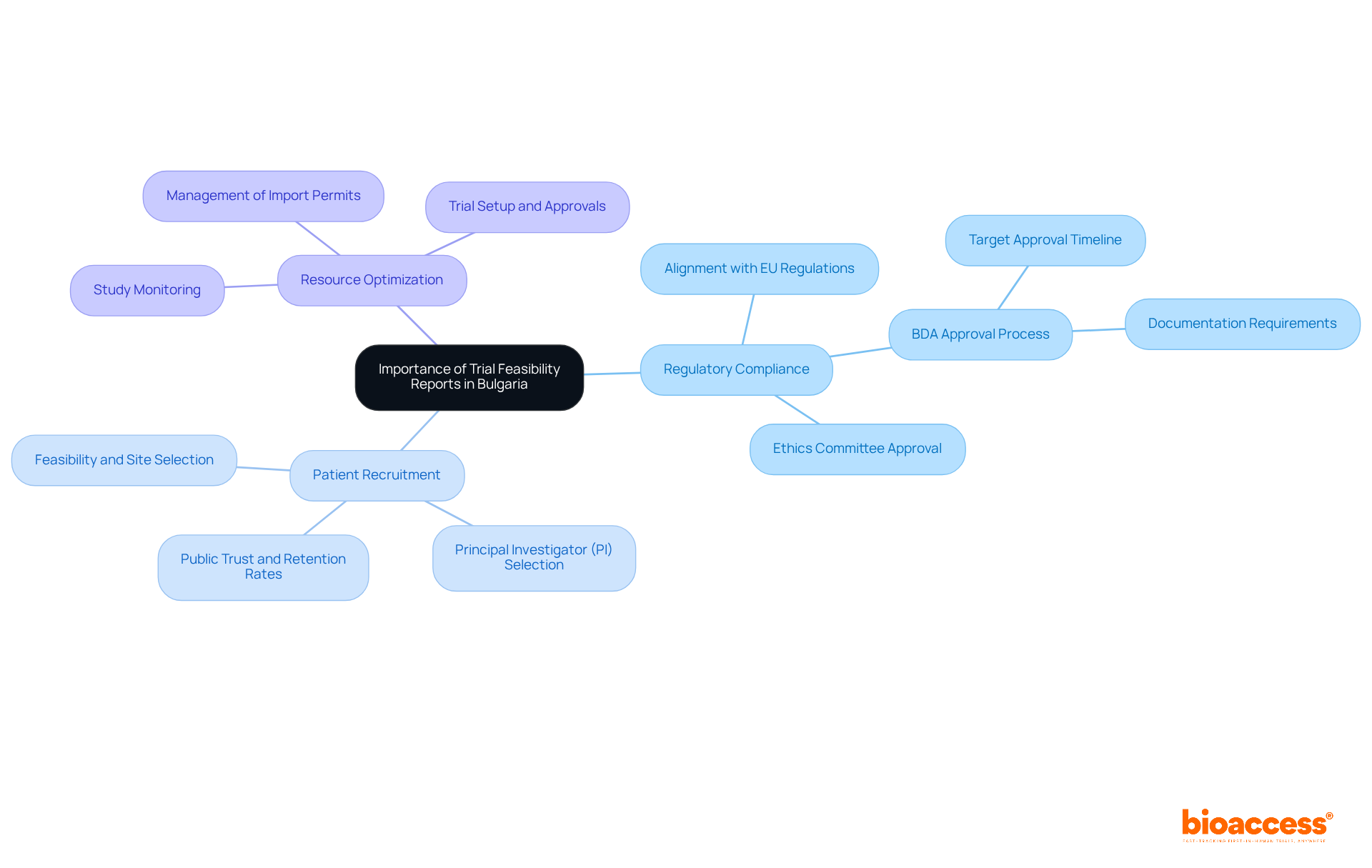
Bulgaria has firmly established itself as a pivotal hub for clinical research in Europe, thanks to its skilled workforce, cost-effective operations, and an efficient regulatory environment. The significance of the requirements for trial feasibility reports in Bulgaria cannot be overstated in this context. As Bulgaria aligns with EU regulations, particularly the Clinical Trials Regulation (EU No 536/2014), the requirements for trial feasibility reports in Bulgaria have become increasingly demanding. These documents are essential for meeting the requirements for trial feasibility reports in Bulgaria, ensuring compliance with local laws, facilitating efficient patient recruitment, and optimizing resource allocation, all of which collectively enhance the likelihood of study success.
Bioaccess plays a crucial role in this process by providing a comprehensive suite of services, including:
With Bulgaria's ongoing commitment to expanding its clinical trials portfolio, understanding the intricacies of feasibility reports is vital for researchers and sponsors. This knowledge is key to navigating the dynamic environment of clinical research successfully.
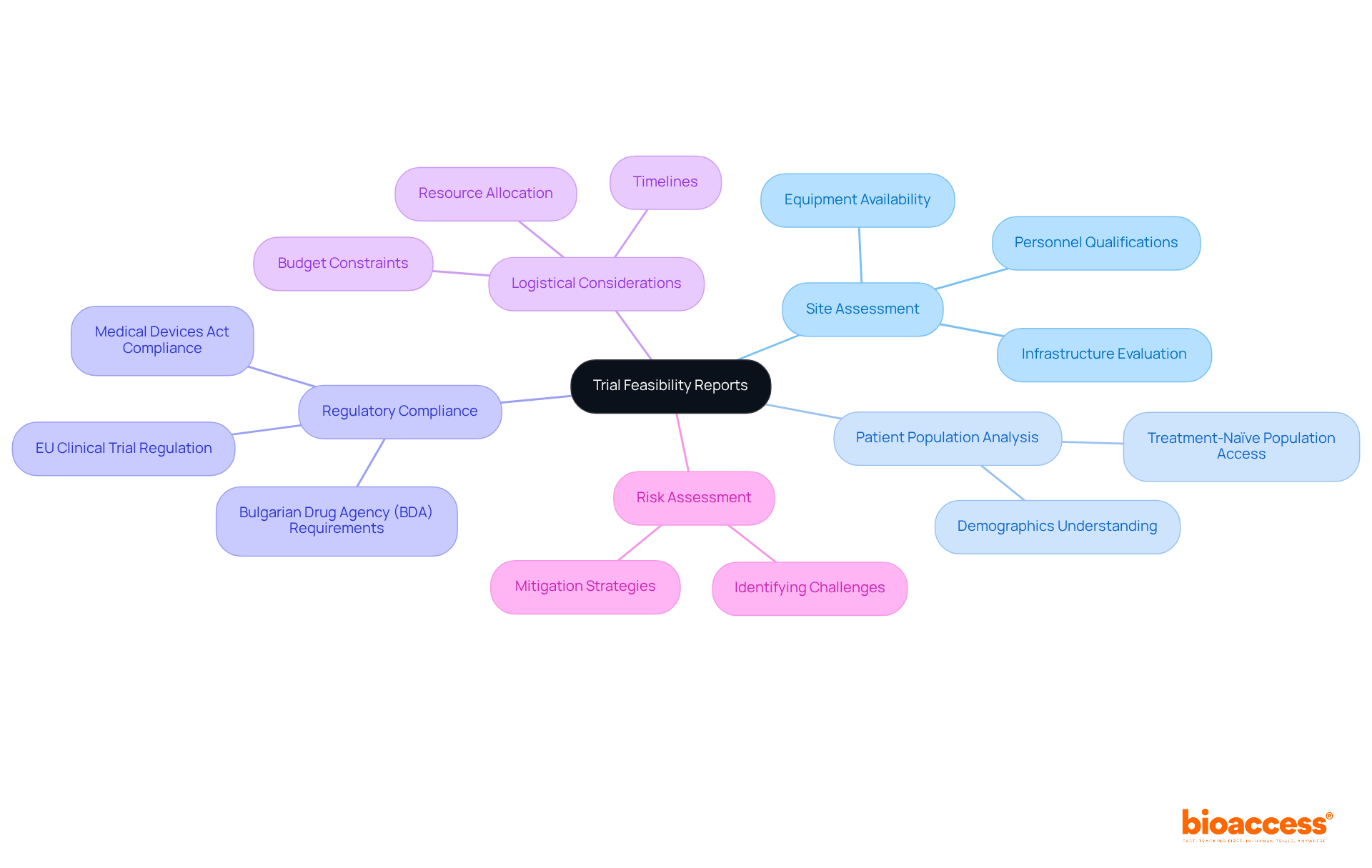
In Bulgaria, trial feasibility reports must encompass several essential components to ensure successful clinical research outcomes:
Site Assessment: A thorough evaluation of the research site's capabilities is crucial. This encompasses evaluating infrastructure, accessible equipment, and the qualifications of the personnel to ensure they fulfill the requirements of the study.
Patient Population Analysis: Understanding the demographics and availability of potential participants is vital for adequate recruitment. This analysis aids in identifying the most appropriate locations based on their access to treatment-naïve populations, which are especially advantageous for clinical studies.
Regulatory Compliance: The report must adhere to both Bulgarian and EU regulations, including the submission of necessary documentation to the Bulgarian Drug Agency (BDA). Compliance with the Medical Devices Act and the EU Clinical Trial Regulation is essential for smooth approval processes.
Logistical Considerations: Operational factors such as timelines, budget constraints, and resource allocation should be addressed. Effective planning in these areas can significantly improve the practicality of the experiment.
Risk Assessment: Identifying potential challenges and outlining strategies to mitigate risks associated with the experiment is critical. This proactive approach can help avoid common pitfalls that lead to costly delays or failed studies.
By integrating these components, assessment documents can offer a thorough summary that aids informed decision-making, ultimately improving the chances of successful execution while meeting the requirements for trial feasibility reports in Bulgaria.
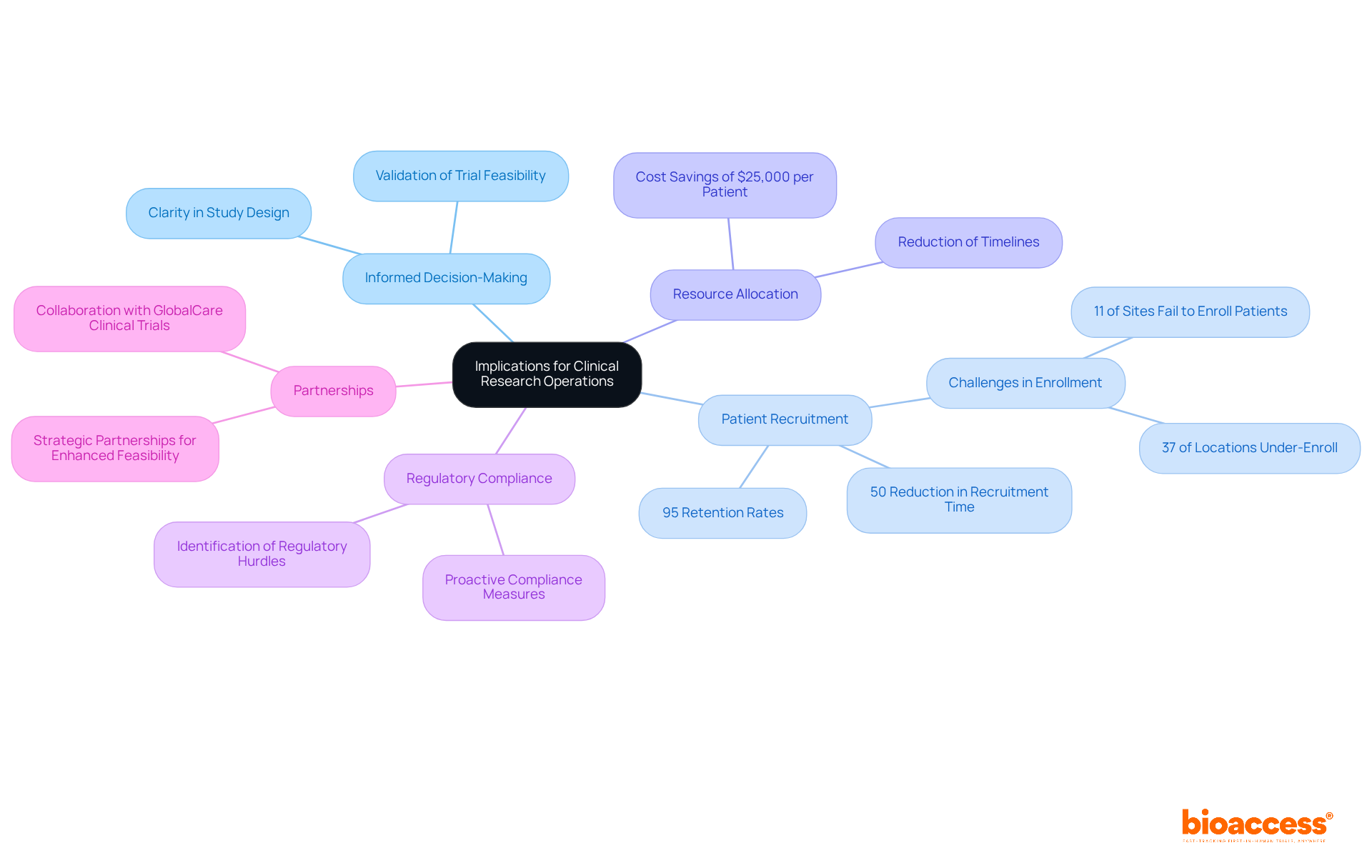
The consequences of feasibility assessments for research activities are profound. By providing a thorough evaluation of site capabilities and patient demographics, these reports empower sponsors to make informed decisions about study locations and designs. This leads to more effective patient recruitment and resource allocation, ultimately reducing timelines and costs associated with research studies. Furthermore, comprehensive evaluations help identify potential regulatory hurdles early in the process, allowing for proactive measures to ensure compliance.
As Bulgaria continues to position itself as a leader in medical research, the requirements for trial feasibility reports in Bulgaria become crucial for maintaining high standards of quality and efficiency in research operations. Notably, partnerships like that of GlobalCare Clinical Trials with bioaccess™ enhance clinical trial ambulatory services, achieving over a 50% reduction in recruitment time and 95% retention rates. This demonstrates the substantial impact of well-managed clinical studies on local economies and healthcare improvement.
Understanding the requirements for trial feasibility reports in Bulgaria is crucial for navigating the complexities of clinical research in this rapidly evolving landscape. These reports not only evaluate the practicality of conducting studies but also ensure compliance with stringent local and EU regulations. As Bulgaria emerges as a significant player in the European clinical research arena, the importance of these assessments cannot be overstated.
The article highlights several critical components of trial feasibility reports:
Each of these elements plays a vital role in enhancing the likelihood of successful study execution and informed decision-making. By addressing these factors, researchers and sponsors can optimize their strategies, leading to more effective patient recruitment and resource allocation.
Given the growing significance of clinical research in Bulgaria, it is increasingly important for stakeholders to prioritize the development of comprehensive trial feasibility reports. These documents serve as a foundation for successful research operations, driving efficiency and quality in clinical studies. Emphasizing meticulous planning and adherence to local practices will empower researchers to harness Bulgaria's potential as a leading hub for medical research, ultimately benefiting both the scientific community and the healthcare landscape.
What are trial feasibility reports?
Trial feasibility reports are documents that evaluate the practicality of conducting a study at a specific location or within a defined area.
What factors are analyzed in feasibility assessments?
Feasibility assessments analyze factors such as site capabilities, patient demographics, regulatory requirements, and logistical considerations.
Why are trial feasibility reports important in Bulgaria?
In Bulgaria, trial feasibility reports are essential to ensure that studies are conducted effectively and ethically, given the rapidly evolving landscape for medical research.
How do feasibility assessments benefit sponsors and researchers?
Feasibility assessments serve as a vital resource for sponsors and researchers, enabling informed decisions regarding study design and execution.
What challenges do feasibility assessments help address?
Feasibility assessments help address potential challenges and ensure adherence to local practices, significantly enhancing the likelihood of successful medical research.