


The article emphasizes the critical benefits and compliance aspects of telemedicine in clinical trials, showcasing its pivotal role in enhancing accessibility, patient engagement, cost efficiency, and data collection. By detailing how telemedicine effectively overcomes geographical barriers, improves communication, reduces costs, and accelerates participant recruitment, it establishes a compelling case for its adoption. Furthermore, it underscores the necessity of adhering to regulatory and ethical standards to safeguard patient privacy and ensure informed consent, thereby reinforcing the importance of these practices in the evolving landscape of clinical research.
Telemedicine is revolutionizing the landscape of clinical trials, offering innovative solutions that transcend geographical barriers and enhance patient engagement. As the healthcare sector shifts towards more patient-centered approaches, the integration of telemedicine streamlines research processes while significantly improving accessibility and data integrity. However, these advancements introduce critical challenges regarding compliance and ethical considerations that researchers must navigate.
How can stakeholders ensure that the benefits of telemedicine are harnessed effectively while maintaining rigorous standards of patient safety and data privacy?
Telemedicine in clinical trials represents a pivotal advancement in healthcare, as it leverages electronic information and communication technologies to deliver services remotely. This innovative approach enhances remote patient observation, virtual consultations, and efficient data collection through telemedicine in clinical trials, thereby significantly improving accessibility and operational efficiency in clinical investigations. By facilitating interaction between researchers and participants regardless of geographical constraints, telemedicine in clinical trials is indispensable for decentralized studies, which broadens participant recruitment and bolsters data integrity. Furthermore, this method aligns with the increasing demand for patient-centered healthcare solutions, rendering studies more inclusive and effective.
As the telehealth sector is projected to experience substantial growth, with an estimated CAGR of 23.8% from 2025 to 2030, the use of telemedicine in clinical trials is set to become more prevalent. This shift will undoubtedly transform the landscape of medical investigations, underscoring the necessity for collaboration and innovation in the field. By embracing telemedicine, researchers can not only streamline their processes but also address key challenges in clinical research, ultimately leading to improved outcomes and patient experiences.

Integrating telemedicine into clinical trials presents a multitude of advantages that significantly enhance the research process:
These benefits not only simplify the research process but also aid in better patient results and contentment, establishing remote healthcare as a revolutionary factor in contemporary medical studies.

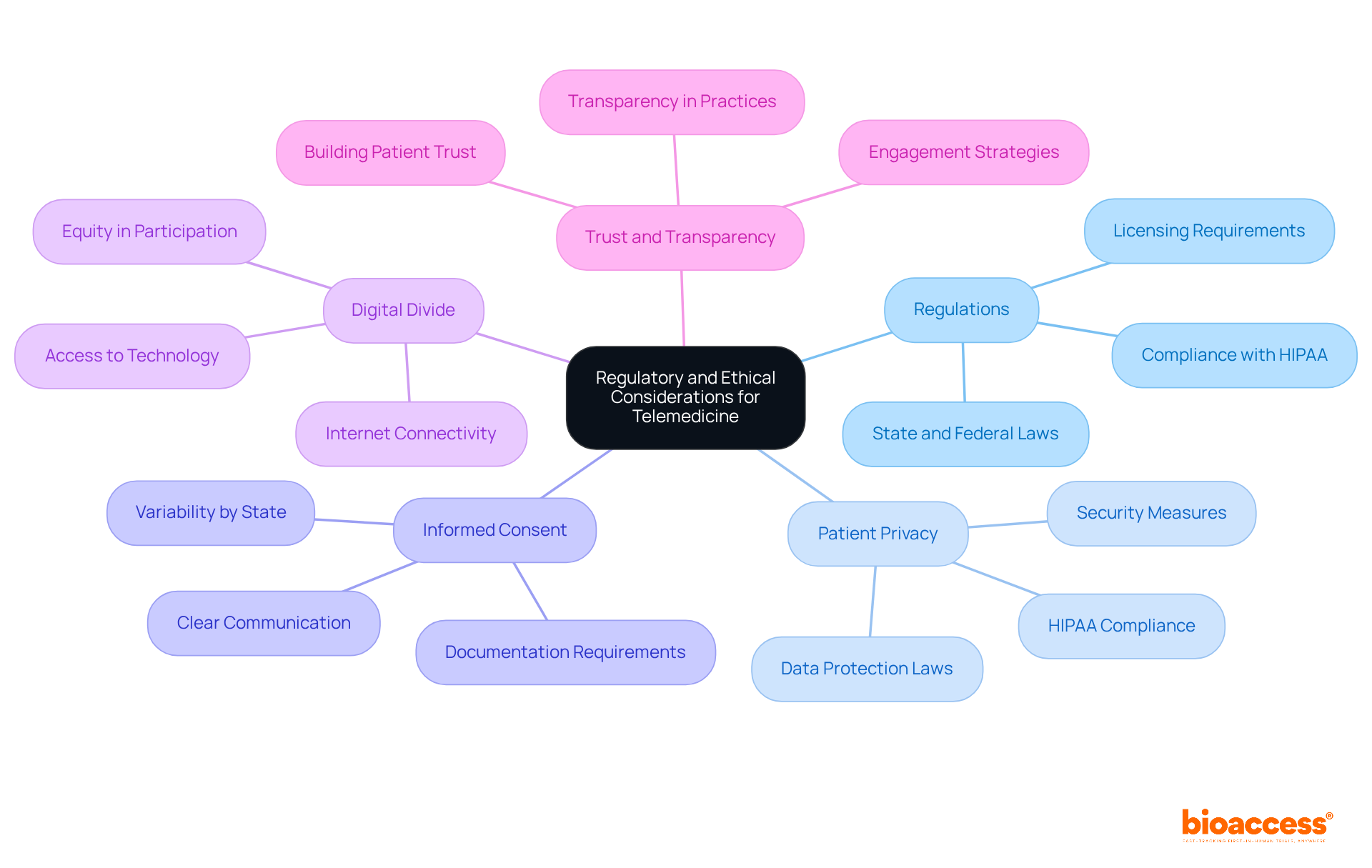
The integration of telemedicine in clinical trials is paramount, demanding meticulous attention to regulatory and ethical considerations. Researchers must navigate a complex landscape of local, state, and federal regulations governing telemedicine, including strict licensing requirements and comprehensive patient consent protocols. Compliance with these regulations is not merely a formality; it is foundational to the integrity of clinical research.
Safeguarding patient information is of utmost importance. Adherence to HIPAA and other data protection laws is essential to protect sensitive health information during remote interactions, ensuring that patient privacy is maintained. This commitment to data privacy not only fulfills legal obligations but also fosters trust among participants.
It is vital that participants fully comprehend the implications of their involvement with telemedicine in clinical trials. Clear communication regarding the use of technology and data handling practices is necessary to uphold ethical standards and ensure informed consent. By prioritizing informed consent, researchers reinforce their commitment to ethical practices, which is crucial for participant engagement and retention.
Researchers must also address the digital divide, ensuring that all potential participants have access to the necessary technology and internet connectivity to engage in telemedicine in clinical trials. This factor is essential for promoting inclusivity and equity in medical studies, as equitable access is a cornerstone of ethical research.
By proactively addressing these factors, clinical trial sponsors can cultivate trust and transparency, ultimately enhancing the credibility and success of their research initiatives. This strategic approach not only aligns with best practices but also positions researchers as leaders in the evolving landscape of clinical trials.
Telemedicine has emerged as a transformative force in clinical trials, fundamentally reshaping the landscape of healthcare research through enhanced remote patient engagement and data collection. This innovative approach not only bolsters accessibility and operational efficiency but also aligns seamlessly with the increasing demand for patient-centered solutions, rendering clinical studies more inclusive and effective.
The integration of telemedicine into clinical trials presents several key advantages:
These benefits collectively streamline the research process, leading to superior patient outcomes and establishing telemedicine as a revolutionary element in modern medical investigations. Moreover, navigating regulatory and ethical considerations is paramount; adherence to laws and transparent communication with participants are essential for maintaining trust and integrity in clinical research.
As the clinical trial landscape evolves, embracing telemedicine is crucial for researchers dedicated to enhancing participant experiences and study outcomes. Prioritizing inclusivity and compliance while leveraging technology will foster collaboration and innovation. By doing so, the full potential of telemedicine in clinical trials can be realized, paving the way for more effective and equitable healthcare solutions in the future.
What is telemedicine in the context of clinical trials?
Telemedicine in clinical trials refers to the use of electronic information and communication technologies to deliver healthcare services remotely, enhancing patient observation, virtual consultations, and data collection.
How does telemedicine improve clinical trials?
Telemedicine improves clinical trials by increasing accessibility, operational efficiency, and participant recruitment while ensuring data integrity, especially in decentralized studies.
Why is telemedicine important for decentralized studies?
Telemedicine is important for decentralized studies because it allows researchers to interact with participants regardless of geographical constraints, broadening recruitment opportunities and enhancing the inclusiveness of studies.
What is the projected growth of the telehealth sector?
The telehealth sector is projected to experience a substantial growth rate, with an estimated compound annual growth rate (CAGR) of 23.8% from 2025 to 2030.
What impact will the growth of telemedicine have on clinical trials?
The growth of telemedicine is expected to transform the landscape of medical investigations by streamlining processes, addressing challenges in clinical research, and ultimately improving outcomes and patient experiences.