


The primary objective of this article is to elucidate the ANVISA fee schedule for medical devices in 2025 and its ramifications for manufacturers pursuing approval in Brazil. It is essential for manufacturers to grasp these fees, which differ based on device classification and can substantially influence approval timelines. This understanding is crucial for effectively navigating the regulatory landscape and ensuring timely market entry.
Navigating the intricate landscape of medical device approval in Brazil demands a keen understanding of the National Health Surveillance Agency's (ANVISA) regulatory framework and fee structures. As manufacturers prepare for the upcoming 2025 fee schedule, grasping the implications of these costs is essential for ensuring compliance and facilitating efficient market entry. However, with the potential for unexpected expenses and delays looming, companies must consider:
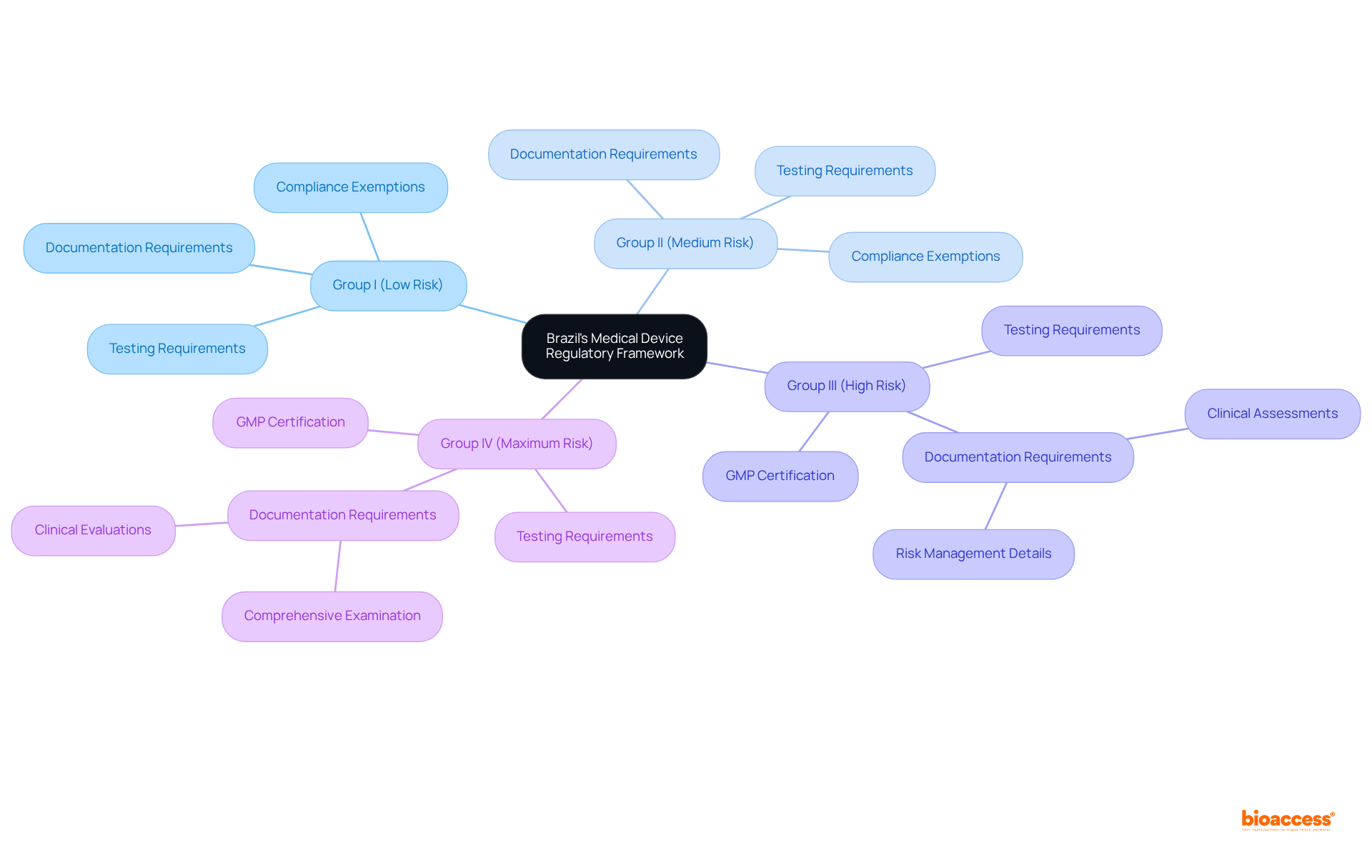
Brazil's medical equipment regulatory framework is primarily overseen by the National Health Surveillance Agency (ANVISA), responsible for ensuring the safety, efficacy, and quality of medical products before they reach the market. This framework categorizes equipment into four groups—Group I, II, III, and IV—based on their risk levels, with Group I representing the lowest risk and Group IV the highest. Each classification comes with specific documentation, testing, and requirements from the anvisa fee schedule 2025 devices, which are essential for manufacturers to grasp, as they directly affect the approval process and associated costs. Notably, Categories III and IV instruments require a comprehensive examination of documentation, including specifications, risk management details, and clinical assessments, crucial for effectively navigating the regulatory environment.
Moreover, registration for Categories III and IV medical equipment is valid for a decade, while producers must renew their Good Manufacturing Practice (GMP) certificate every two years to maintain registration validity. In contrast, Class I and II devices are exempt from Brazil Good Manufacturing Practice (BGMP) requirements, underscoring the varying levels of regulatory scrutiny across device categories. Recent updates to ANVISA regulations, particularly with the introduction of RDC No. 751/2022, have brought significant changes to the classification and validation processes. This regulation aligns more closely with international standards, including the EU Medical Device Regulation, thereby facilitating smoother market access for manufacturers.
Compliance with quality management system standards, such as ISO 13485, is often critical for expediting approvals and ensuring adherence to ANVISA's stringent requirements. At bioaccess®, our team, including regulatory affairs experts like Katherine Ruiz and Ana Criado, is committed to guiding Medtech startups through these intricate regulatory landscapes, ensuring they achieve timely market access and compliance with both Brazilian and Colombian regulations.
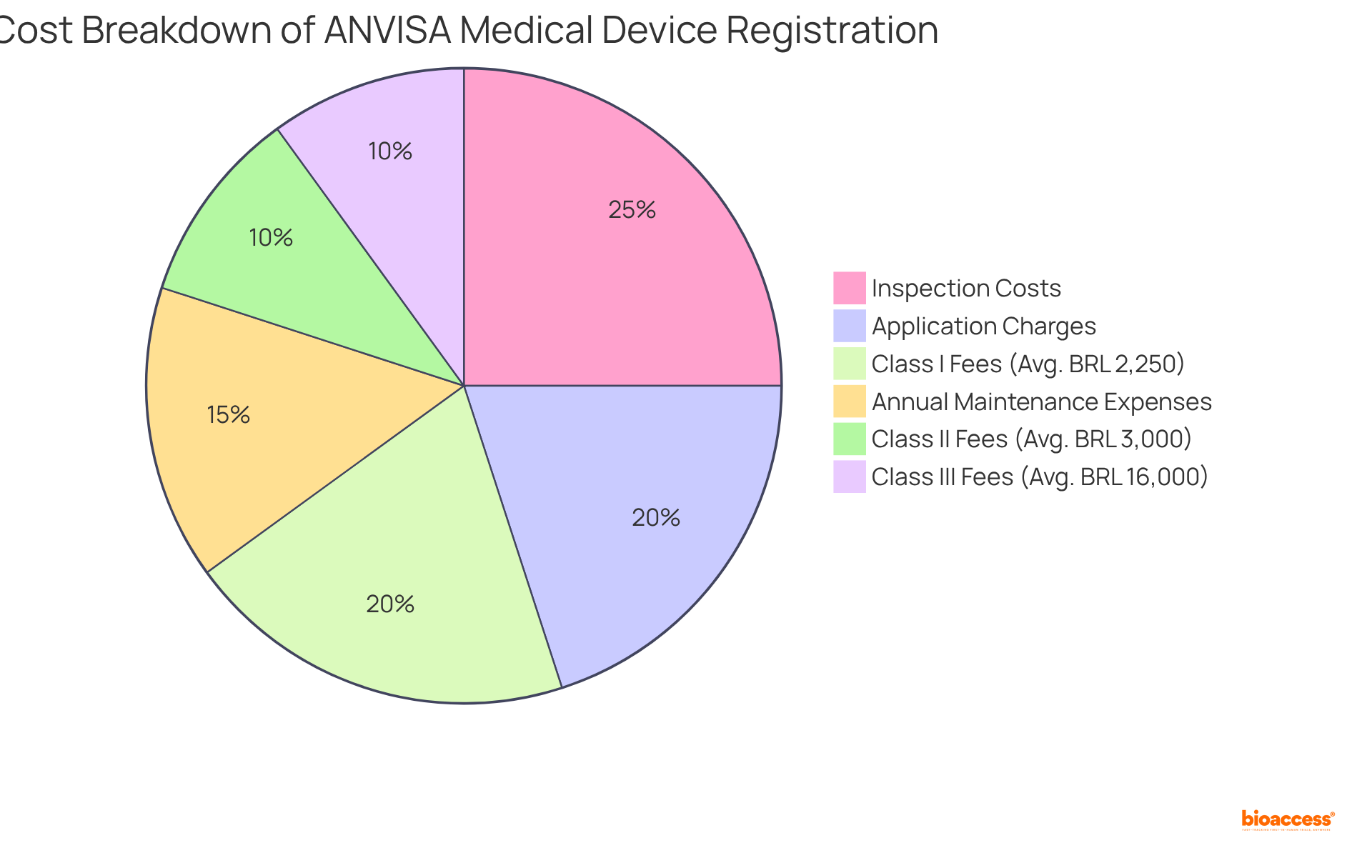
The ANVISA pricing framework for medical equipment registration is a critical component for producers in the Medtech landscape. It encompasses several elements, including:
These charges differ according to device classification; for example, Class I devices generally incur lower costs compared to Class III devices, which necessitate more extensive evaluations and testing. Furthermore, it is essential for producers to account for costs related to the submission of technical documentation and any required clinical trials. Understanding these charges early in the development process is vital to circumvent unexpected expenses and delays.
Given that ANVISA periodically updates the anvisa fee schedule 2025 devices, it is crucial to stay informed about these changes for compliance and effective financial planning. According to the anvisa fee schedule 2025 devices, registration charges for Class I medical instruments in 2025 range from BRL 1,500 to BRL 3,000, while Notificação charges can fluctuate between R$2,000 and R$4,000, and Registro charges may escalate to approximately R$16,000. Organizations that adeptly manage these charges can streamline their regulatory procedures and enhance their market entry strategies.
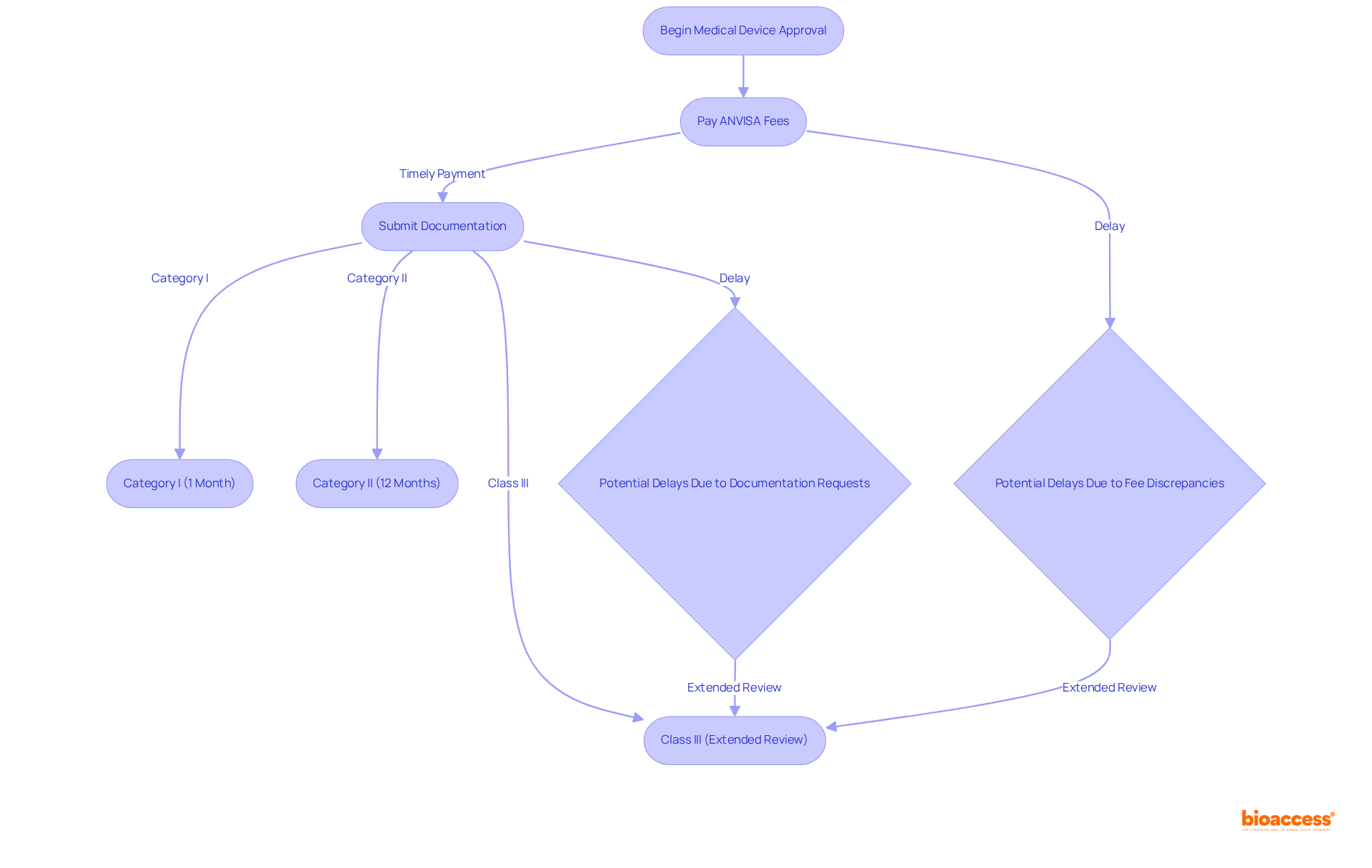
ANVISA fees are pivotal in determining the timelines for the authorization of medical equipment in Brazil. Delays in fee payments or discrepancies in submissions can substantially prolong review periods, consequently hindering market entry. For instance, the approval time for Category I items typically spans about one month, while Category II items may require approximately 12 months. In contrast, Class III products often demand a more comprehensive review process, which can extend timelines due to the intricate documentation and testing involved.
Manufacturers must remain vigilant in ensuring that all fees are paid promptly and accurately to avert unnecessary delays. The evaluation procedure for advanced equipment necessitates thorough documentation, including clinical assessments and risk analyses, which can further prolong authorization timelines. For example, the standard authorization duration for Class III products can significantly increase if issues arise with fee submissions or if ANVISA requests additional documentation.
Expert opinions underscore the importance of comprehending the ANVISA fee schedule 2025 devices and associated timelines, which enables manufacturers to establish realistic expectations for product launches. By proactively managing fee payments and familiarizing themselves with the typical authorization timelines for each device category, manufacturers can navigate the regulatory landscape more effectively concerning the ANVISA fee schedule 2025 devices, enhancing their prospects for timely market entry. Additionally, ethical authorizations in Brazil take 4-6 weeks, a critical factor to consider within the overall timeline for market entry. Insights from regulatory experts, such as Ana Criado, can further support manufacturers in devising effective strategies for managing the approval process and ensuring compliance with ANVISA requirements.
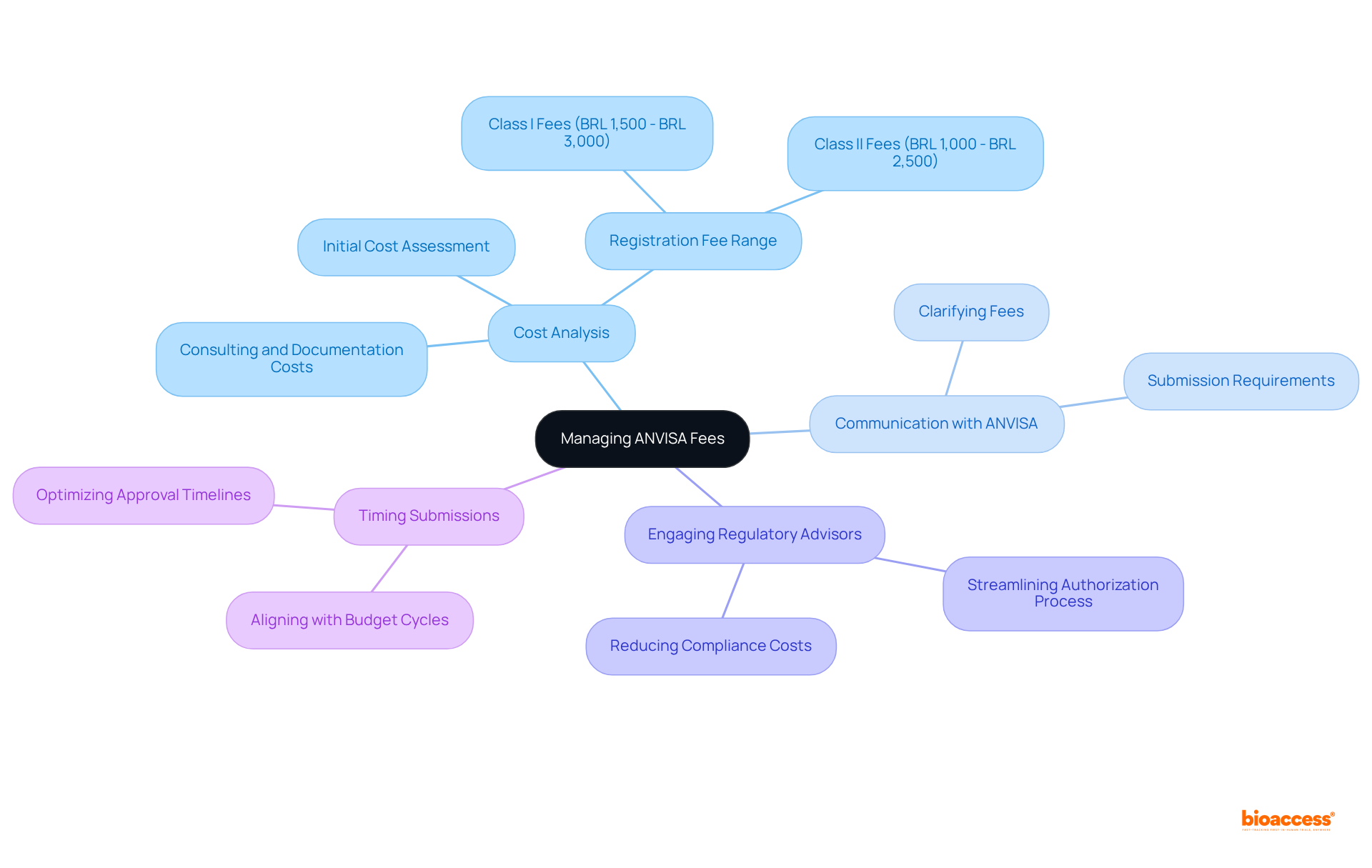
To efficiently manage ANVISA charges, manufacturers must implement a variety of strategic approaches.
By employing these strategies, companies can navigate the financial intricacies of medical device development more effectively, ultimately improving their prospects for securing timely approvals.
Navigating the ANVISA fee schedule for medical devices in 2025 is essential for manufacturers seeking timely approvals within Brazil's competitive Medtech environment. A comprehensive understanding of the regulatory framework and associated costs is vital for ensuring compliance and facilitating efficient market entry. The categorization of devices into Groups I through IV underscores the varying levels of scrutiny and corresponding fees, highlighting the necessity for thorough preparation and strategic planning.
Insights from the article reveal that the ANVISA fee structure significantly influences the approval timelines for medical devices. Delays in fee payments or discrepancies in submissions can result in extended review periods, necessitating proactive management by manufacturers. By conducting detailed cost analyses, maintaining open lines of communication with ANVISA, and consulting regulatory experts, companies can streamline their processes and improve their chances of achieving timely market access.
Ultimately, effectively managing ANVISA fees not only supports compliance but also plays a crucial role in the success of medical device development in Brazil. Manufacturers are urged to remain informed about the evolving fee schedule and to adopt strategic approaches that align with their financial planning. By doing so, they can adeptly navigate the complexities of the regulatory environment and position themselves advantageously for future opportunities within the medical device market.
What is the primary agency responsible for regulating medical devices in Brazil?
The primary agency responsible for regulating medical devices in Brazil is the National Health Surveillance Agency (ANVISA).
How are medical devices categorized in Brazil's regulatory framework?
Medical devices in Brazil are categorized into four groups—Group I, II, III, and IV—based on their risk levels, with Group I representing the lowest risk and Group IV the highest.
What are the specific requirements for Categories III and IV medical devices?
Categories III and IV medical devices require a comprehensive examination of documentation, including specifications, risk management details, and clinical assessments.
How long is the registration valid for Categories III and IV medical equipment?
The registration for Categories III and IV medical equipment is valid for a decade.
How often must producers renew their Good Manufacturing Practice (GMP) certificate?
Producers must renew their Good Manufacturing Practice (GMP) certificate every two years to maintain registration validity.
Are Class I and II devices subject to Good Manufacturing Practice (GMP) requirements?
No, Class I and II devices are exempt from Brazil Good Manufacturing Practice (BGMP) requirements.
What significant changes were introduced by RDC No. 751/2022?
RDC No. 751/2022 introduced significant changes to the classification and validation processes, aligning more closely with international standards, including the EU Medical Device Regulation.
Why is compliance with quality management system standards like ISO 13485 important?
Compliance with quality management system standards, such as ISO 13485, is critical for expediting approvals and ensuring adherence to ANVISA's stringent requirements.
How can Medtech startups navigate Brazil's regulatory landscape?
Medtech startups can navigate Brazil's regulatory landscape with the assistance of regulatory affairs experts, such as those at bioaccess®, who provide guidance for timely market access and compliance with regulations.