


This article examines the critical differences between radiopharmaceutical and biopharmaceutical trials, highlighting their unique regulatory frameworks, trial designs, and patient recruitment strategies.
It is essential to understand how radiopharmaceuticals are governed by regulations specific to their radioactive properties, often employing adaptive trial designs that cater to their unique challenges.
In contrast, biopharmaceuticals adhere to different guidelines and typically utilize randomized controlled studies.
This distinction underscores the necessity for tailored approaches in clinical research to effectively address the complexities presented by each category.
The realms of radiopharmaceuticals and biopharmaceuticals are rapidly evolving, each playing a pivotal role in modern medicine. As the demand for innovative therapies surges, understanding the nuances between these two categories becomes essential for navigating clinical trials effectively. Researchers face significant challenges when designing studies that adhere to the distinct regulatory frameworks and trial methodologies of radiopharma and biopharma.
What specific obstacles must be overcome to ensure compliance and efficacy? This exploration delves into the critical differences in trial designs, patient recruitment strategies, and success metrics, offering insights that could shape the future of therapeutic development.
Radiopharmaceuticals are specialized drugs that incorporate radioactive isotopes, serving both diagnostic and therapeutic roles. Primarily utilized in imaging examinations, such as PET scans, they also play a critical role in targeted cancer therapies, administering radiation directly to impacted tissues while minimizing damage to adjacent healthy cells. For instance, the use of iodine-131 in treating thyroid cancer exemplifies the effectiveness of radiopharmaceuticals in clinical practice.
In contrast, biopharmaceuticals, commonly referred to as biologics, are derived from biological sources, including proteins, nucleic acids, or living cells. These products are pivotal in treating a range of diseases, particularly autoimmune disorders and various cancers. The monoclonal antibodies segment, which accounted for a significant market share of 31% in 2024, illustrates the growing reliance on biopharmaceuticals for effective treatment options.
Understanding the difference between radiopharma and biopharma trials is crucial for navigating the complexities present in clinical trials within each field. Recent data indicates that the global biopharmaceuticals market is projected to grow from USD 666.41 billion in 2025 to USD 1,183.87 billion by 2032, reflecting the increasing demand for innovative therapies. Industry leaders stress the significance of understanding both nuclear medicines and biopharmaceuticals, as progress in these fields is essential for enhancing patient outcomes and addressing unfulfilled medical requirements.
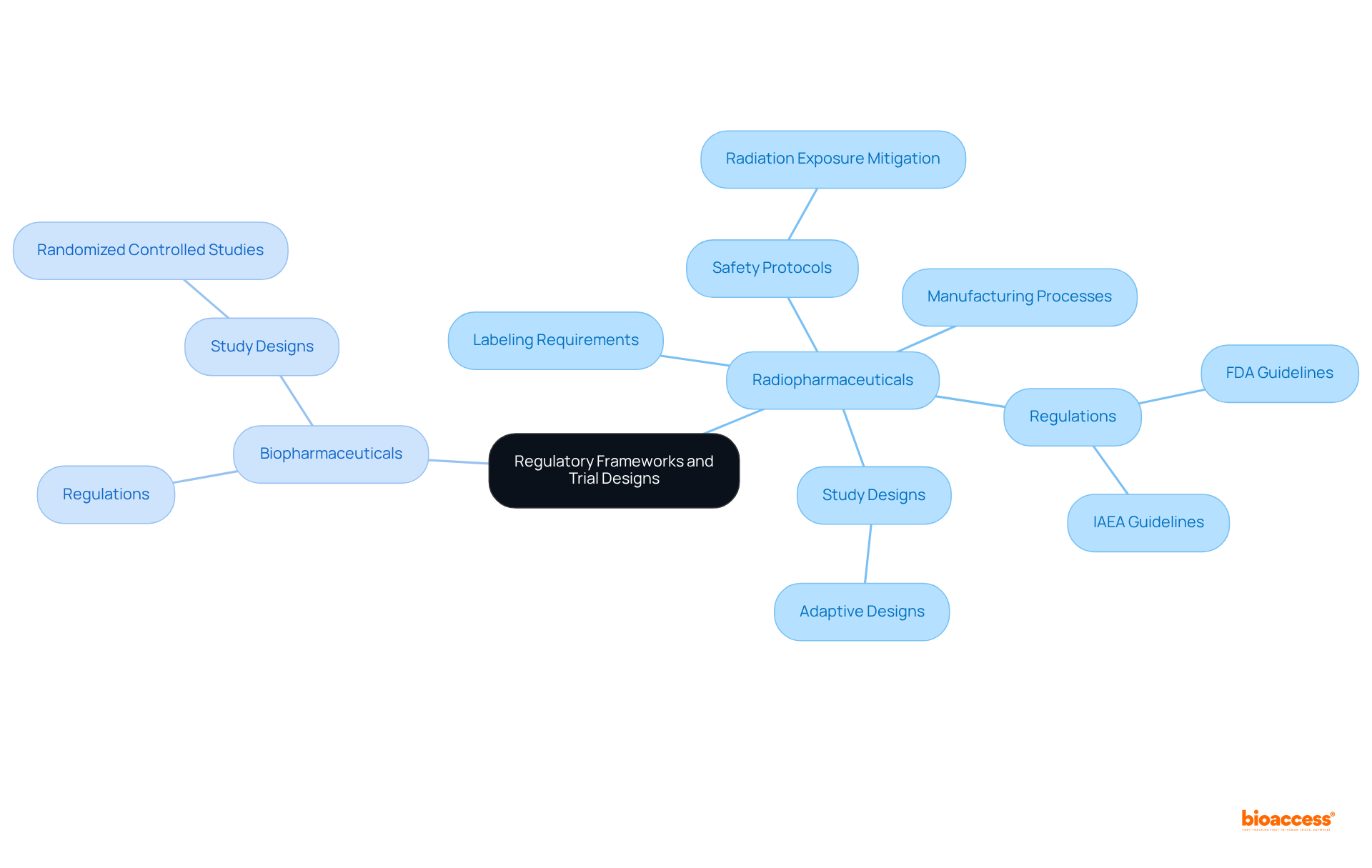
The regulatory frameworks governing radiopharmaceuticals and biopharmaceuticals exhibit notable differences, highlighting the difference between radiopharma and biopharma trials, primarily due to the unique characteristics inherent to each category. Radiopharmaceuticals are subject to rigorous regulations stemming from their radioactive properties, necessitating strict adherence to guidelines established by the FDA and the International Atomic Energy Agency (IAEA). These regulations encompass critical aspects such as:
Conversely, biopharmaceuticals are regulated under distinct guidelines that emphasize the biological processes involved in their development. This divergence in regulatory focus leads to variations in study designs. Radiopharmaceutical studies frequently utilize adaptive designs, permitting adjustments based on real-time data and the particular challenges presented by radiation. In contrast, biopharmaceutical studies typically employ randomized controlled studies, which are essential for assessing the efficacy and safety of therapeutic agents.
Comprehending these regulatory frameworks and the difference between radiopharma and biopharma trials is essential for navigating the intricacies of clinical research in both areas. Hospitals serve as the primary end-users in the market for medical isotopes, playing a crucial role in the application of these therapies. As the landscape of medical innovation evolves, staying updated on the latest FDA guidelines and IAEA regulations will empower researchers to conduct effective and compliant studies. Moreover, insights from regulatory specialists at bioaccess® can offer valuable perspectives on enhancing study designs for radioactive pharmaceuticals, ensuring that startups can expedite their clinical research outcomes and navigate the regulatory environment with assurance. Bioaccess® provides vital services such as regulatory approval and participant recruitment, which are critical for effectively executing studies in these complex settings.
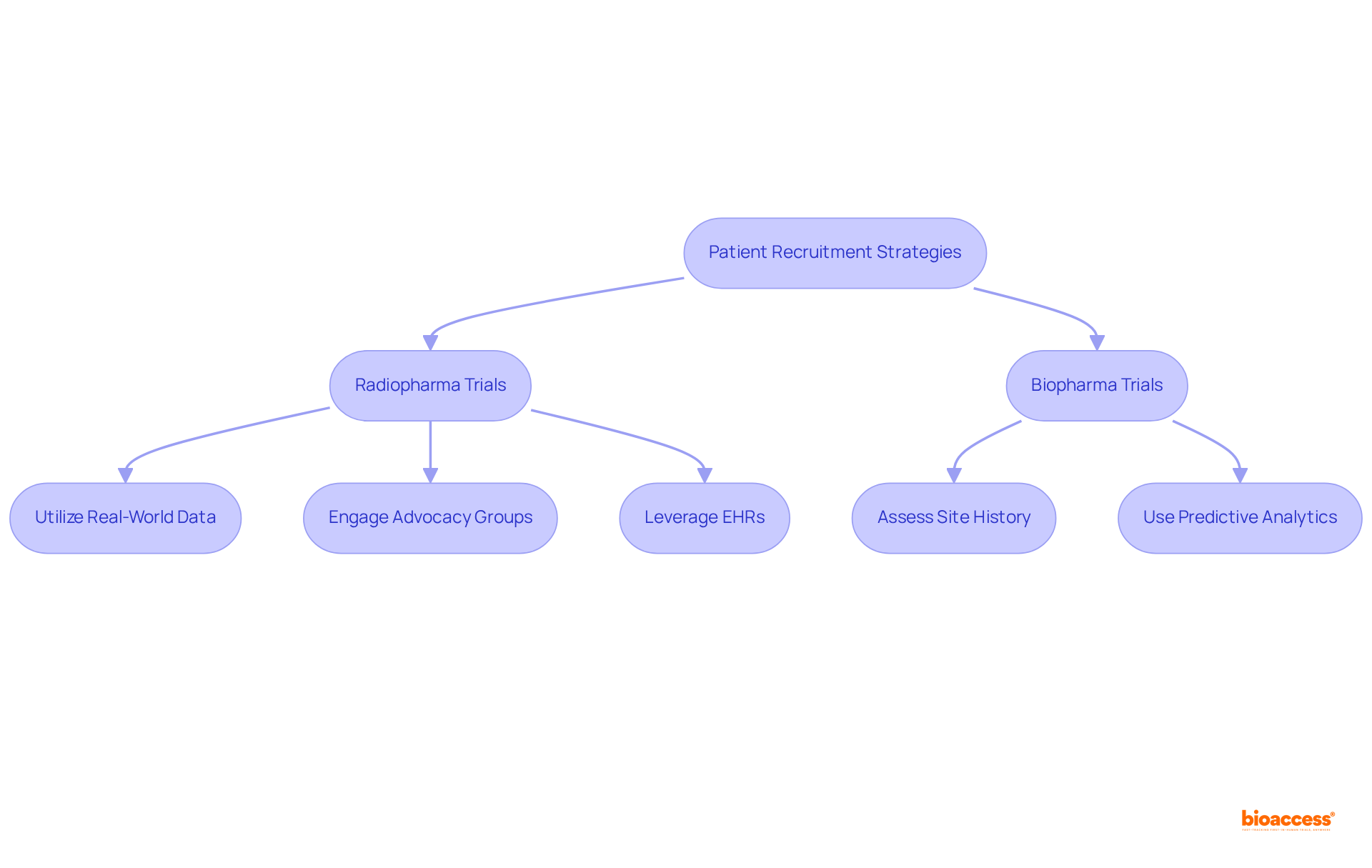
Efficient participant recruitment techniques must be tailored to the distinct attributes of each domain, reflecting the difference between radiopharma and biopharma trials. For radiopharmaceutical trials, utilizing real-world data and electronic health records (EHRs) significantly enhances the identification of potential candidates, streamlining the recruitment process. Involvement with advocacy groups is essential, as it promotes outreach initiatives and establishes trust within communities.
Site selection plays a pivotal role in recruitment success. Selecting locations with a demonstrated history of successful participant enrollment and skilled researchers results in enhanced outcomes. Predictive analytics can assess site performance and patient demographics, enabling sponsors to make informed choices about where to conduct studies. This data-driven method ensures that studies are located in environments supportive of achieving recruitment objectives, ultimately enhancing the integrity and validity of the research.
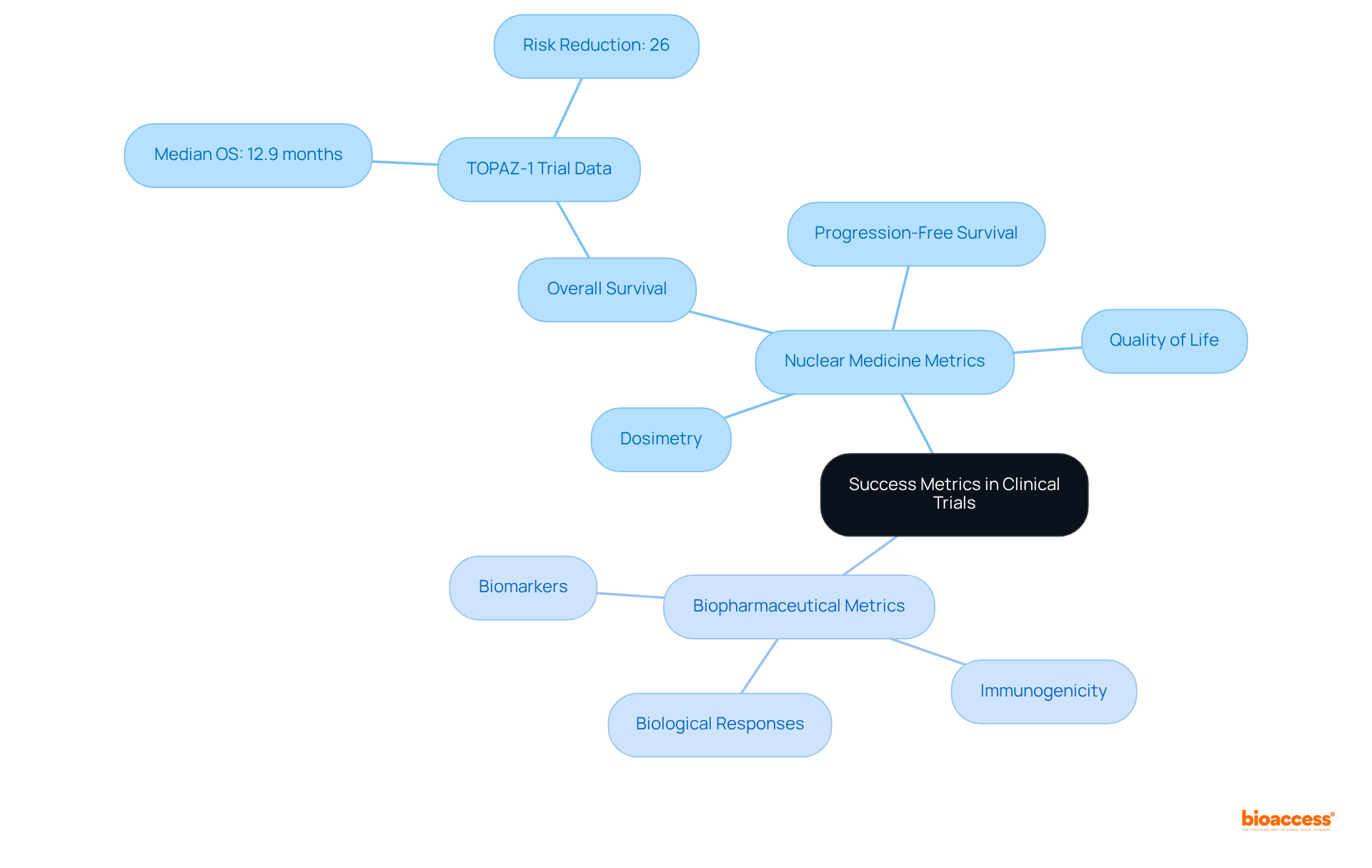
Success metrics for nuclear medicine and biopharmaceutical studies encompass a range of critical endpoints, including overall survival, progression-free survival, and quality of life measures. In radiopharmaceutical studies, additional metrics often focus on dosimetry and the effectiveness of targeted radiation delivery, which are essential for evaluating therapeutic accuracy and individual outcomes. Conversely, biopharmaceutical studies typically emphasize biological responses to interventions, such as biomarkers and immunogenicity, which provide insights into the intervention's mechanism of action and potential efficacy.
Recent data underscores the importance of these metrics. For instance, the TOPAZ-1 trial demonstrated a median overall survival of 12.9 months for individuals receiving a combination of Imfinzi and chemotherapy, compared to 11.3 months for those undergoing chemotherapy alone. This 26% reduction in the risk of death highlights the significance of overall survival as a primary objective in assessing success. As Do-Youn Oh, MD, PhD, noted, "The latest data from TOPAZ-1 indicate that twice as many individuals with advanced biliary tract cancer were still alive at three years with durvalumab and chemotherapy, an especially significant advance in a setting where historically the prognosis has been poor."
Analyzing these outcomes not only aids in determining the efficacy of treatments but also informs future research directions and regulatory decisions. For stakeholders, grasping these metrics is vital for evaluating the success of clinical trials and their implications for patient care, ultimately guiding the development of innovative therapies that can significantly enhance patient outcomes.
Understanding the distinctions between radiopharmaceuticals and biopharmaceuticals is crucial for navigating the complexities of clinical trials in these fields. Both types of pharmaceuticals play significant roles in modern medicine; however, their unique characteristics and regulatory requirements necessitate tailored approaches to trial design, patient recruitment, and outcome evaluation.
Key insights from the article highlight the differences in:
Radiopharmaceuticals are governed by stringent regulations due to their radioactive nature, contrasting with the biologically focused guidelines for biopharmaceuticals. Moreover, the article discusses the importance of adaptive trial designs in radiopharmaceutical studies compared to the randomized controlled studies commonly employed in biopharmaceutical research. Effective patient recruitment strategies and site selection are pivotal in ensuring successful trial outcomes, with data-driven approaches enhancing recruitment efficiency.
The significance of this understanding extends beyond clinical trials, impacting the development of innovative therapies that can improve patient outcomes. As the biopharmaceutical market continues to grow, staying informed about the latest trends and regulatory standards in both radiopharmaceutical and biopharmaceutical trials is essential. By fostering collaboration and leveraging insights from regulatory specialists, stakeholders can navigate the complexities of these trials, ultimately leading to advancements in treatment options and better health outcomes for patients.
What are radiopharmaceuticals?
Radiopharmaceuticals are specialized drugs that incorporate radioactive isotopes, used for both diagnostic and therapeutic purposes, primarily in imaging examinations like PET scans and targeted cancer therapies.
How do radiopharmaceuticals work in cancer treatment?
They administer radiation directly to affected tissues, such as in the case of iodine-131 for treating thyroid cancer, while minimizing damage to surrounding healthy cells.
What are biopharmaceuticals?
Biopharmaceuticals, or biologics, are drugs derived from biological sources such as proteins, nucleic acids, or living cells, and are crucial in treating diseases, particularly autoimmune disorders and various cancers.
What is the significance of monoclonal antibodies in biopharmaceuticals?
Monoclonal antibodies represent a significant segment of the biopharmaceutical market, accounting for 31% in 2024, highlighting their importance in effective treatment options.
Why is understanding the difference between radiopharmaceuticals and biopharmaceuticals important?
Understanding the differences is crucial for navigating the complexities of clinical trials in each field, which can impact patient outcomes and medical advancements.
What is the projected growth of the biopharmaceuticals market?
The global biopharmaceuticals market is projected to grow from USD 666.41 billion in 2025 to USD 1,183.87 billion by 2032, indicating a rising demand for innovative therapies.
Why is progress in radiopharmaceuticals and biopharmaceuticals significant?
Progress in these fields is essential for enhancing patient outcomes and addressing unmet medical needs, as emphasized by industry leaders.