


The article elucidates the critical distinction between side effects and adverse effects, emphasizing their inherent nature and predictability.
For instance, the predictable drowsiness associated with antihistamines serves as a clear example of a side effect, in stark contrast to the unpredictable and potentially life-threatening allergic reactions that fall under adverse effects.
This differentiation underscores the necessity of comprehending these terms, which is vital for ensuring patient safety and fostering effective clinical practice.
The realm of medicine is laden with complexities, particularly when navigating the nuanced distinctions between side effects and adverse effects of medications. Grasping these differences is not merely an academic exercise; it holds significant implications for patient safety and treatment efficacy. As healthcare professionals confront the challenge of effectively managing these reactions, one must consider: how can a clearer understanding of side effects and adverse effects enhance clinical outcomes and patient adherence? This article delves into these critical concepts, exploring their definitions, mechanisms, and the vital role they play in the healthcare landscape.
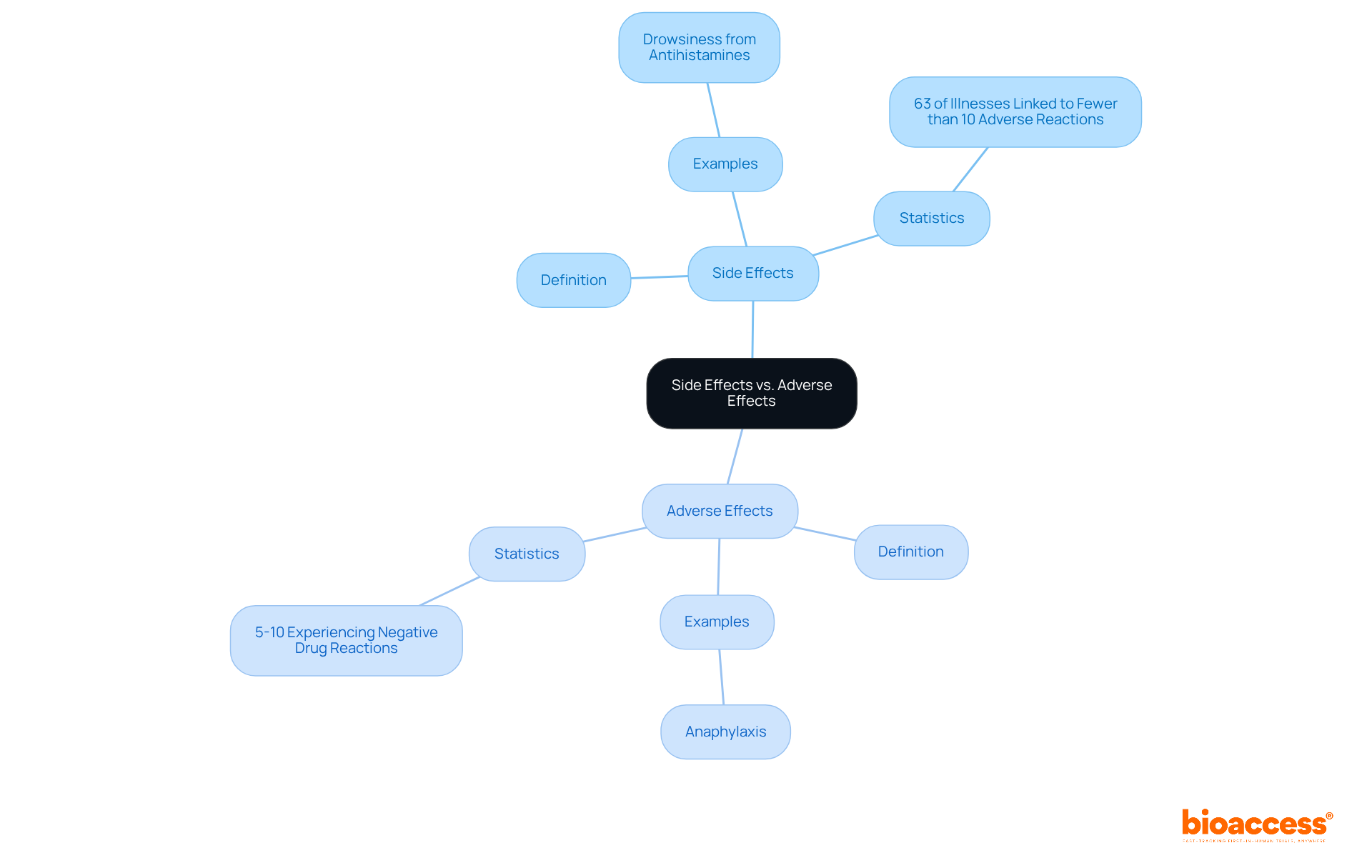
Side reactions represent unintended results of a medication that occur alongside the desired therapeutic outcomes. These outcomes can be either advantageous or detrimental, and they are often predictable based on the drug's pharmacological characteristics. For instance, drowsiness is a common consequence associated with antihistamines, typically anticipated due to their mechanism of action. Recent studies indicate that approximately 63% of illnesses are linked to fewer than 10 adverse reactions, while only 4 diseases are associated with over 100 adverse reactions, highlighting the variability in adverse reaction profiles among different medications.
In contrast, negative outcomes specifically refer to harmful or unwanted reactions that arise from a medication or intervention. These outcomes are generally unforeseen and can pose significant health risks. For example, an allergic reaction to a medication, such as anaphylaxis, is classified as a negative outcome. Research suggests that around 5% to 10% of individuals may experience a negative drug reaction (ADR) upon admission, during hospitalization, or at discharge. Notably, one in four individuals on long-term medication has encountered at least one ADR over a six-year period, underscoring the critical need for monitoring and managing these risks.
Understanding the differences between side effect and adverse effect is essential for healthcare professionals and researchers, as it facilitates accurate assessment and reporting of clinical outcomes. Specialist perspectives emphasize the importance of ongoing education regarding the potential for both reactions and negative outcomes, particularly in populations utilizing multiple medications, where the risk of ADRs significantly increases. This knowledge is vital for enhancing patient safety and optimizing therapeutic strategies.
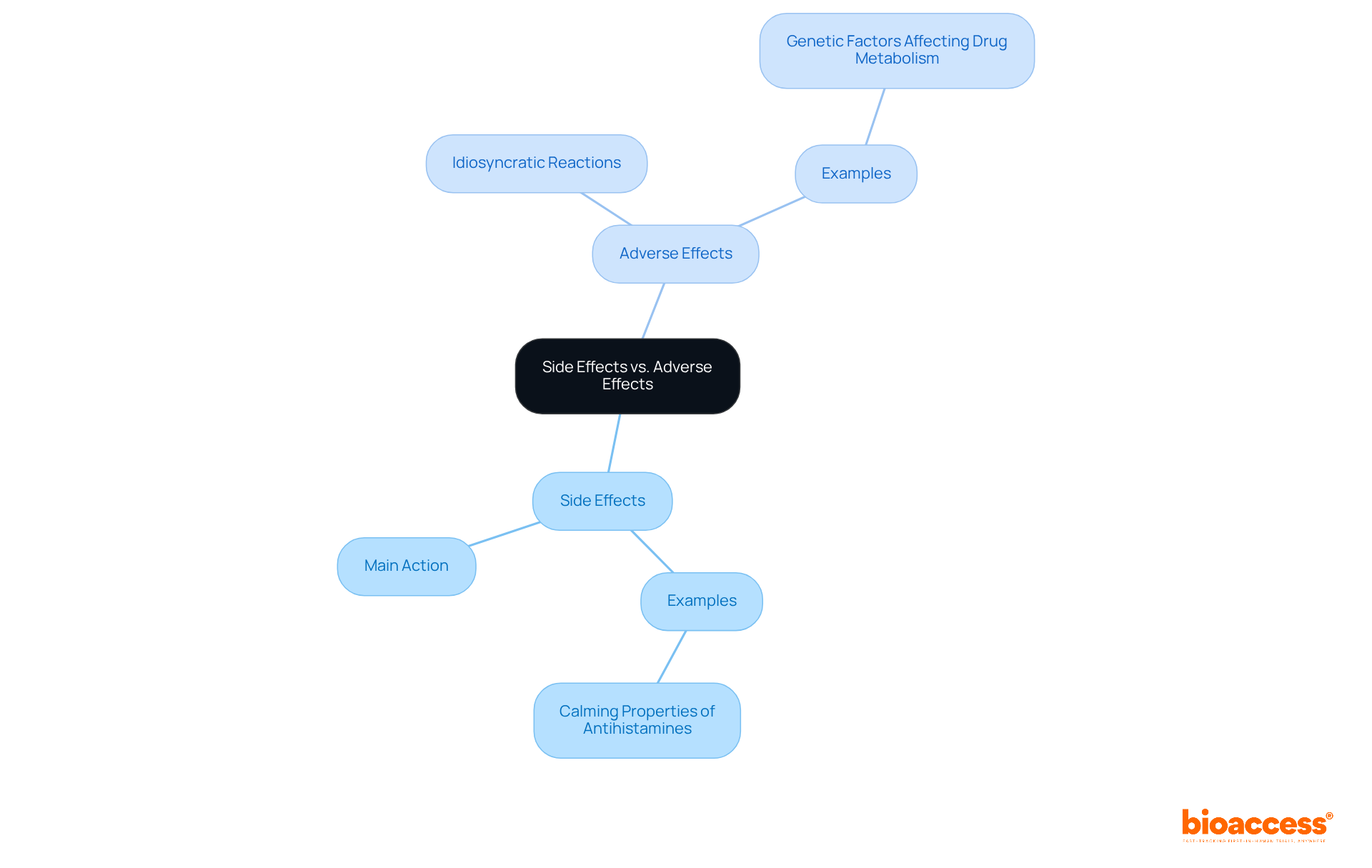
The processes of side reactions and negative reactions can illustrate the differences between side effect and adverse effect, varying significantly based on the medication and the individual. Side reactions frequently develop from the drug's main action on the body, such as the calming properties of antihistamines. In contrast, negative outcomes may arise from idiosyncratic reactions, where the medication interacts unpredictably with the individual's unique biological makeup.
For instance, certain medications may cause liver damage in susceptible individuals due to genetic factors that affect drug metabolism. Understanding the differences between side effect and adverse effect is essential for researchers who aim to create safer clinical trials and for clinicians making informed choices regarding patient care. This knowledge not only enhances the safety of treatments but also underscores the importance of personalized medicine in clinical research.
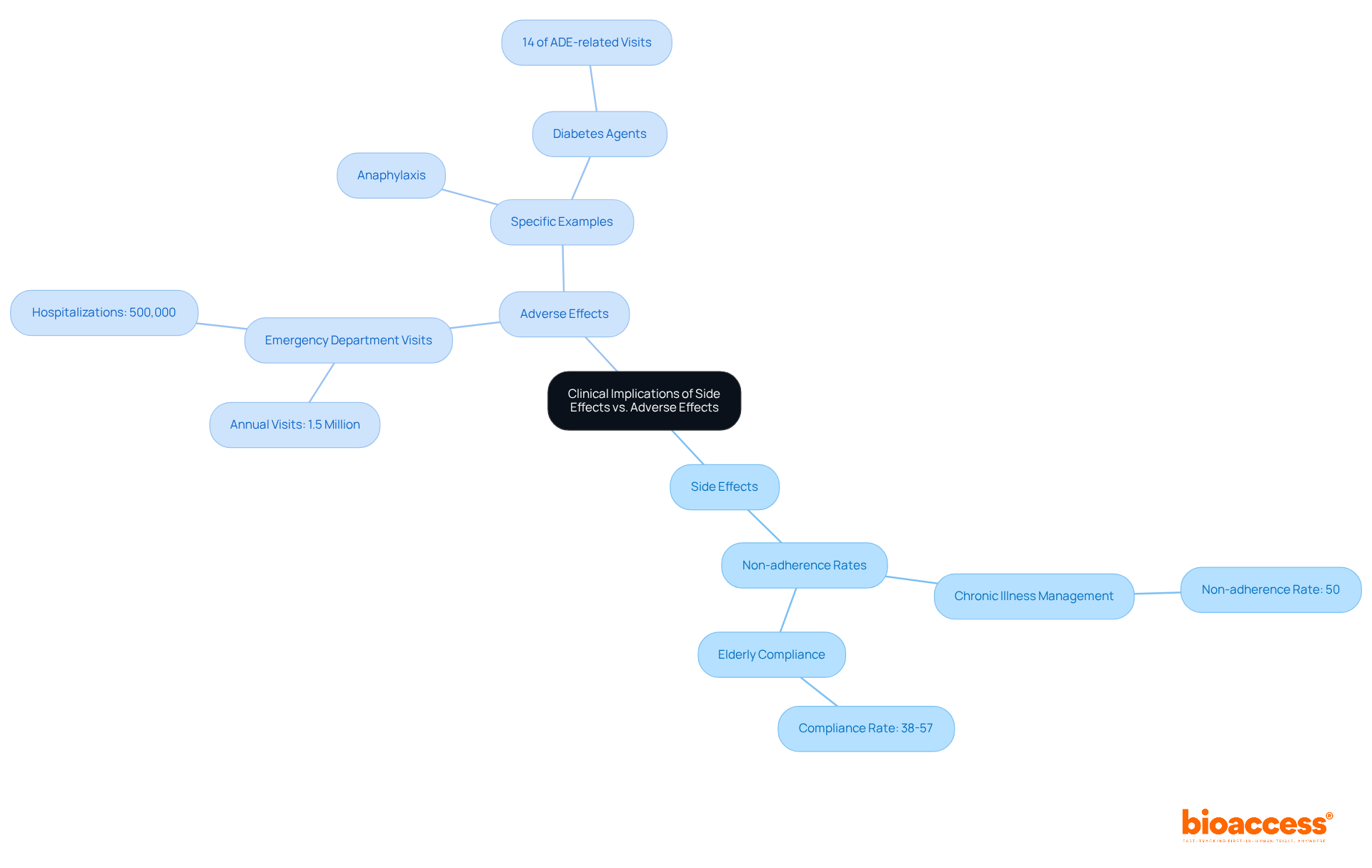
The clinical implications of adverse reactions are profound and multifaceted. Adverse reactions, while often controllable, can significantly impact adherence to treatment plans and the overall effectiveness of therapies. For instance, nausea, a common side effect of numerous medications, can deter individuals from adhering to their prescribed treatments, leading to non-adherence rates that may plummet to as low as 50% in chronic illness management. Particularly concerning are elderly patients, who exhibit compliance rates ranging from 38% to 57%, averaging less than 45%, thereby underscoring the challenges this demographic faces.
In contrast, negative outcomes can precipitate serious health issues that necessitate urgent medical attention. Each year, over 1.5 million individuals in the United States visit emergency departments due to adverse drug events (ADEs), with nearly 500,000 requiring hospitalization. A striking example is anaphylaxis, a life-threatening reaction that can manifest within minutes of drug exposure, demanding immediate treatment to avert fatal consequences. Furthermore, diabetes agents such as insulin account for nearly 14% of emergency department visits related to ADEs, emphasizing the critical need for vigilant monitoring of specific medications.
Understanding the differences between side effect and adverse effect is crucial for healthcare professionals. This knowledge empowers them to implement effective monitoring strategies and health education initiatives, ultimately enhancing safety and treatment efficacy. Moreover, proactively addressing frequent adverse reactions can bolster adherence rates; research indicates that 77% of individuals are inclined to follow treatments aimed at curing illnesses, compared to just 63% for preventive strategies. Additionally, more than 60% of individuals misinterpret medication instructions immediately after consulting their physicians, highlighting the urgent need for clear communication regarding potential adverse reactions and treatment expectations. This underscores the vital importance of developing supportive care strategies to mitigate the impact of adverse reactions on patient compliance.
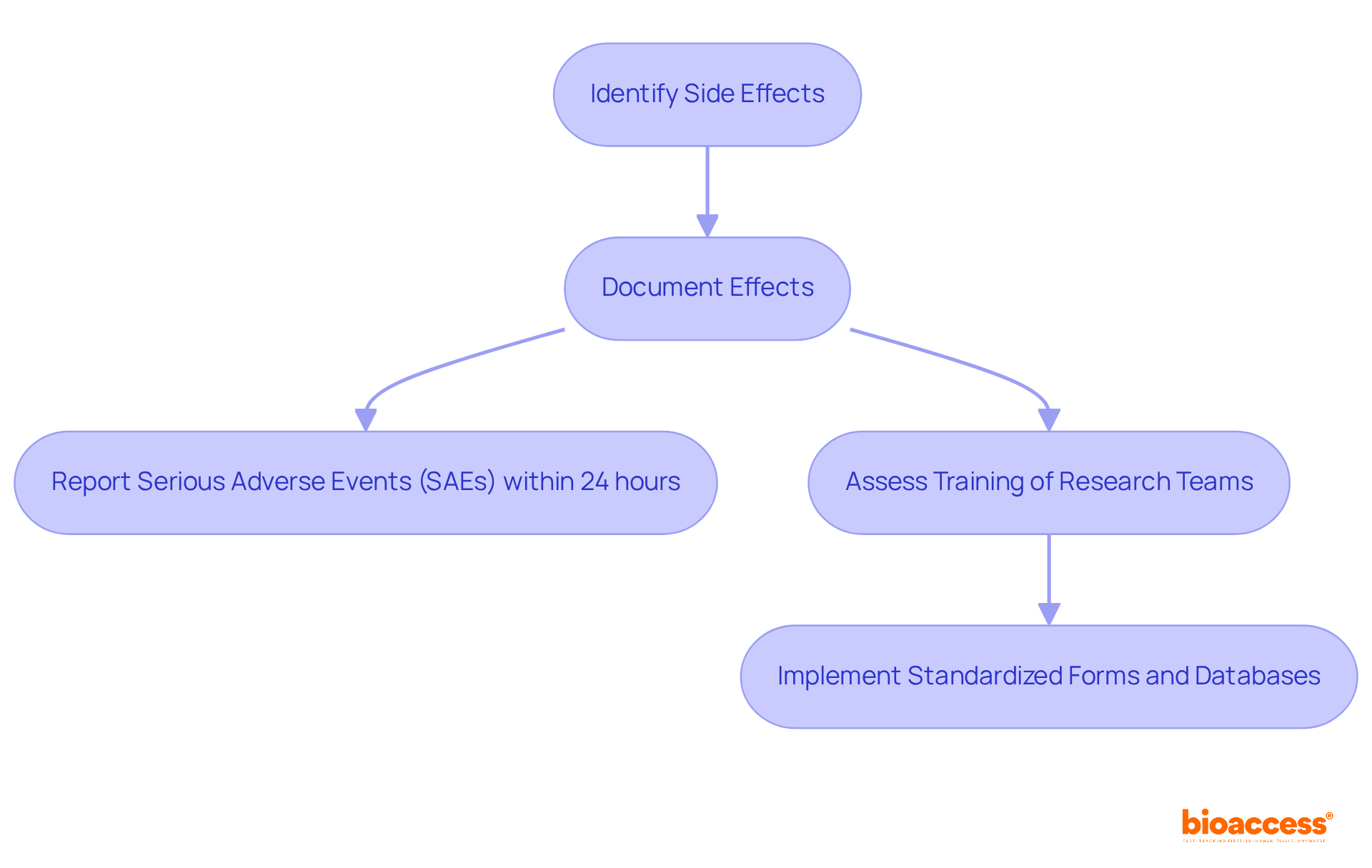
In clinical research, establishing robust systems for identifying and documenting side reactions and negative outcomes is paramount. Researchers must ensure their teams are thoroughly trained to recognize these effects promptly and accurately. Adherence to regulatory standards, such as those from the FDA and EMA, is critical; for example, serious adverse events (SAEs) must be reported within 24 hours of the investigator's awareness of the event.
A recent review highlighted that only 31% of clinical trial reports detailed planned analyses for negative events in their methods sections, revealing a substantial gap in adherence to best practices. Furthermore, the documentation of negative occurrences is often inconsistent, with 82% of studies failing to provide both the count of negative events and the number of individuals affected.
To enhance adherence and ensure data integrity, researchers should implement standardized forms and databases for monitoring negative events. This systematic approach not only protects participants but also deepens the overall understanding of the safety and efficacy of new treatments. Expert recommendations underscore the necessity for clear definitions of safety analysis populations and the adoption of advanced statistical methods to refine the analysis of adverse events, ultimately fostering improved patient care and informed decision-making in clinical trials.
Understanding the distinctions between side effects and adverse effects is crucial for both healthcare professionals and patients. Side effects may be anticipated outcomes accompanying the desired effects of a medication, whereas adverse effects denote harmful and often unpredictable reactions that can pose significant health risks. Grasping these differences enables individuals to navigate their treatment options more effectively and contribute to safer clinical practices.
This article outlines various aspects of side effects and adverse effects, emphasizing the mechanisms behind these reactions and their clinical implications. It highlights the necessity of ongoing education for healthcare providers to ensure proper assessment and management of these effects, particularly in populations more vulnerable to adverse drug reactions. The data presented underscores the need for vigilance in monitoring and reporting these effects to enhance patient safety and treatment adherence.
Ultimately, fostering a comprehensive understanding of side effects and adverse effects is vital for improving patient outcomes. Healthcare professionals are urged to prioritize clear communication regarding potential risks associated with medications and to implement robust monitoring systems in clinical research. By doing so, they can significantly mitigate the impact of these reactions on patient care, ensuring that therapeutic strategies are both effective and safe.
What are side effects in the context of medication?
Side effects are unintended results of a medication that occur alongside the desired therapeutic outcomes. They can be either advantageous or detrimental and are often predictable based on the drug's pharmacological characteristics.
Can you give an example of a common side effect?
An example of a common side effect is drowsiness, which is typically associated with antihistamines due to their mechanism of action.
How prevalent are adverse reactions in illnesses?
Recent studies indicate that approximately 63% of illnesses are linked to fewer than 10 adverse reactions, while only 4 diseases are associated with over 100 adverse reactions, highlighting variability among different medications.
What are negative outcomes in medication use?
Negative outcomes refer to harmful or unwanted reactions that arise from a medication or intervention, which are generally unforeseen and can pose significant health risks.
Can you provide an example of a negative outcome?
An example of a negative outcome is an allergic reaction to a medication, such as anaphylaxis.
How common are negative drug reactions (ADRs)?
Research suggests that around 5% to 10% of individuals may experience a negative drug reaction (ADR) upon admission, during hospitalization, or at discharge. Additionally, one in four individuals on long-term medication has encountered at least one ADR over a six-year period.
Why is it important to understand the differences between side effects and adverse effects?
Understanding the differences is essential for healthcare professionals and researchers as it facilitates accurate assessment and reporting of clinical outcomes, enhances patient safety, and optimizes therapeutic strategies.
What is emphasized regarding education on side effects and adverse reactions?
Ongoing education regarding the potential for both reactions and negative outcomes is emphasized, particularly in populations utilizing multiple medications, where the risk of ADRs significantly increases.