


The article highlights the critical role of Investigational New Drug (IND) applications in clinical research, outlining the essential components and regulatory guidelines necessary for submission to the FDA. A successful IND application demands:
These elements collectively enhance the likelihood of approval and facilitate the safe development of new drugs. Understanding the intricacies of IND applications is paramount for researchers aiming to navigate the complexities of the regulatory landscape effectively.
Navigating the complex landscape of drug development necessitates a profound understanding of regulatory processes, with particular emphasis on the Investigational New Drug (IND) application. This essential submission to the FDA transcends mere bureaucratic formality; it serves as a pivotal gateway that determines whether a promising new treatment can advance to human trials. By grasping the nuances of the IND process, researchers and sponsors can significantly enhance their chances of success, thereby contributing to the progression of medical science.
However, given the continually evolving regulations and the complexities involved, what key strategies can be employed to ensure a seamless IND submission and avert common pitfalls?
An Investigational New Drug (IND) request represents a formal submission to the U.S. Food and Drug Administration (FDA) aimed at obtaining approval to initiate trials for a new drug or biologic in human subjects. This tool is pivotal in the drug development process, encompassing essential data from preclinical studies, detailed manufacturing information, and a proposed clinical trial protocol. The IND submission serves to ensure that the proposed studies are both safe and scientifically valid, thereby safeguarding the rights and welfare of trial participants.
To be deemed suitable for approval, the IND request must convincingly demonstrate that the drug is reasonably safe for initial human use. This is achieved through a thorough evaluation of the data provided, including results from animal studies and information on the drug's pharmacology and toxicology. In 2025, the FDA observed a notable rise in the number of IND requests submitted, reflecting a growing interest in drug development. Current statistics indicate that the approval rates for IND submissions have remained robust, with a significant percentage receiving favorable outcomes.
Key components of an IND abbreviation medical submission include:
These documents must outline the clinical trial design, the Chemistry, Manufacturing, and Controls (CMC) strategy, and any preliminary efficacy data. Additionally, a Letter of Authorization (LOA) may be required for referencing existing IND information. The FDA has released guidance documents that outline the IND submission process, highlighting the significance of adhering to regulatory guidelines while allowing flexibility in the submission approach. This adaptability is crucial, enabling sponsors to navigate the complexities of drug development effectively. Furthermore, the FDA's updates in 2025 concerning the IND submission process emphasize the necessity for sponsors to remain aware of evolving regulatory expectations.
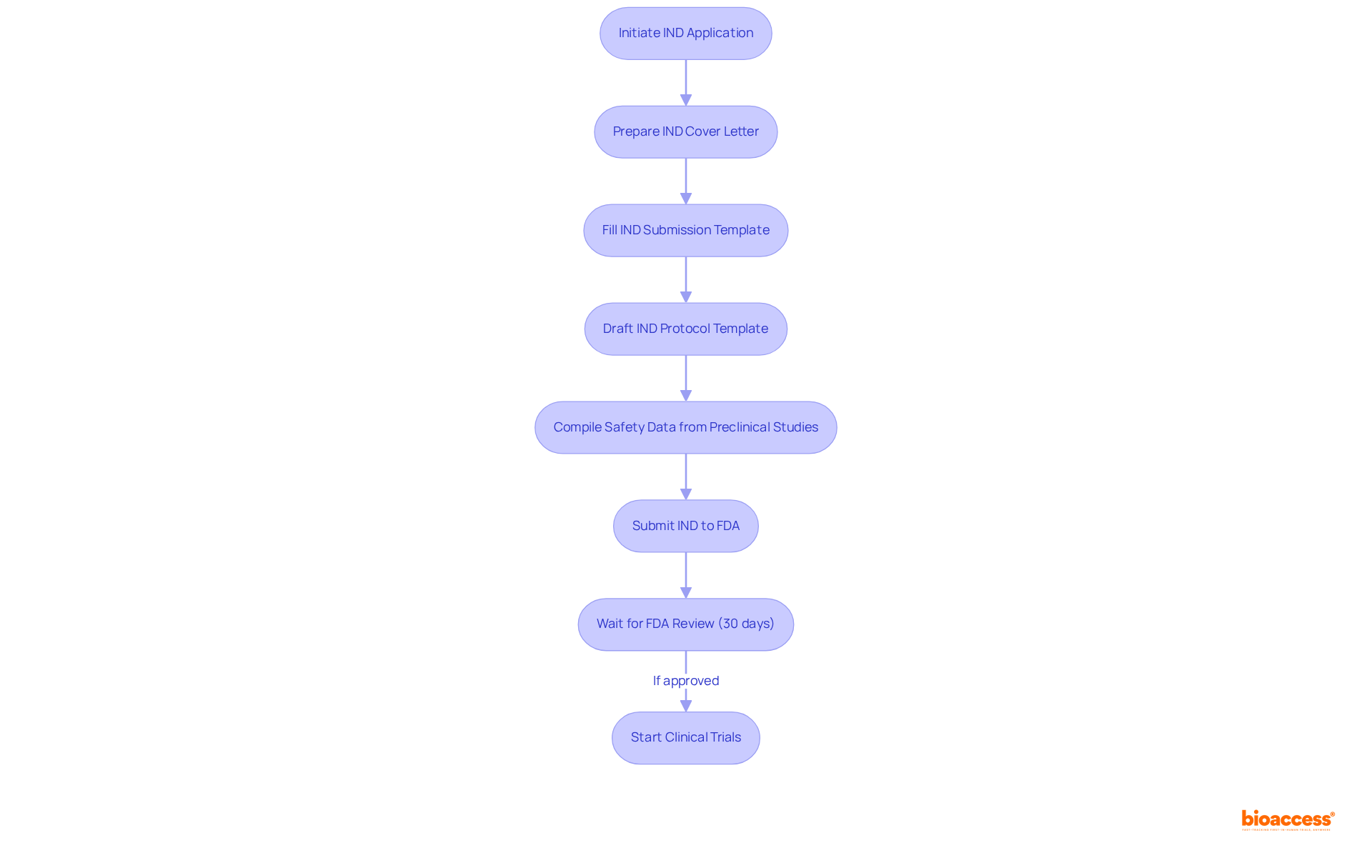
The regulatory guidelines for IND abbreviation medical applications are primarily outlined by the FDA and encompass several key components that are crucial for compliance in clinical research. These guidelines stipulate that the IND must contain:
Understanding these guidelines is essential for ensuring that the submission with the IND abbreviation medical adheres to regulatory standards, thereby facilitating authorization for trials involving patients.
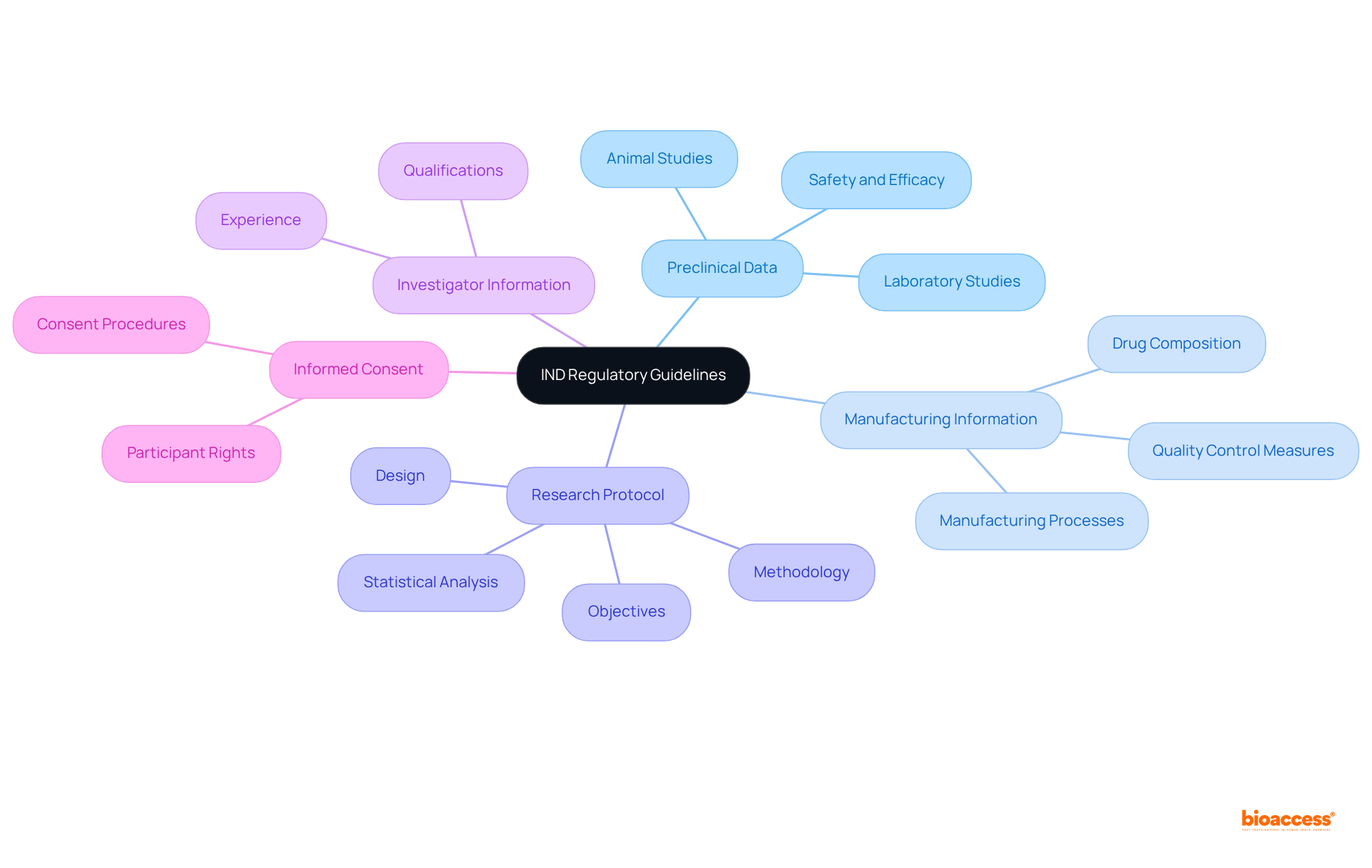
Preparing and submitting an IND abbreviation medical application is a critical process that involves several essential steps.
The success rate of IND submissions is closely linked to the quality of the trial protocol and the thoroughness of the preclinical information provided, as indicated by the IND abbreviation medical. Following best practices in compiling and presenting this information can enhance the likelihood of a favorable review. For instance, a well-documented submission under the IND abbreviation medical can enhance the likelihood of successful FDA approval, enabling timely advancement into clinical trials. Significantly, ethical approvals are provided in 4-6 weeks by bioaccess®, highlighting the efficiency of the IND procedure. A pertinent case study involves Grifols, which obtained FDA approval for its IND request to commence a Phase 2 trial of immunoglobulin drops for dry eye disease, demonstrating a successful IND submission process. Furthermore, it is crucial to acknowledge that the average time needed to prepare an IND submission can differ, but following these steps can simplify the process.
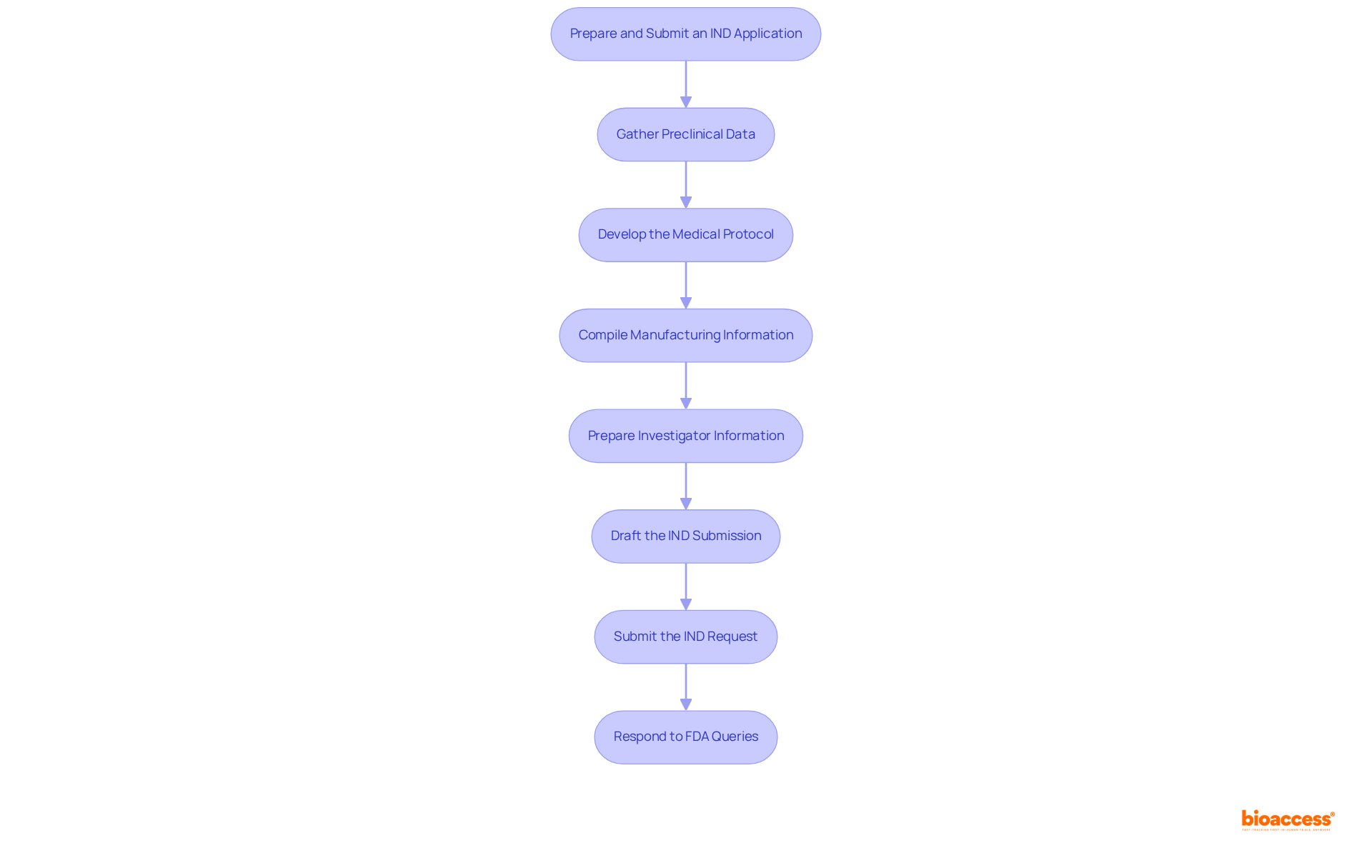
Pre-IND consultation programs are essential for sponsors aiming to enhance their IND submissions by obtaining targeted feedback on trial designs and regulatory strategies, particularly regarding the ind abbreviation medical. To effectively leverage these programs, consider the following steps:
Engaging in pre-IND abbreviation medical consultations not only helps in identifying potential issues early but also fosters a constructive relationship with the FDA. In 2025, an increasing number of sponsors are recognizing the value of these consultations, leading to more successful IND applications. For instance, companies that have effectively utilized pre-IND meetings have reported smoother submission processes and reduced clinical holds, underscoring the importance of thorough preparation and strategic engagement with regulatory bodies.
The Investigational New Drug (IND) abbreviation is pivotal in the clinical research landscape, acting as a gateway for new drugs to enter human trials. A comprehensive understanding of the IND application process, from initial submission to regulatory compliance, is essential for any sponsor aiming to successfully navigate the complexities of drug development.
Key points emphasized throughout the article include:
Moreover, the importance of adhering to FDA guidelines and engaging in pre-IND consultations cannot be overstated, as these steps significantly enhance the likelihood of a favorable review and timely approval. The rise in IND submissions in 2025 reflects a growing commitment to advancing medical research and innovation.
In summary, the significance of the IND application process transcends mere regulatory requirements; it embodies a commitment to patient safety and scientific integrity in drug development. Sponsors are encouraged to leverage available resources, including pre-IND consultations, to optimize their submissions and cultivate a productive relationship with regulatory authorities. By prioritizing these practices, the journey from concept to clinical trial can become more efficient, ultimately leading to the introduction of groundbreaking therapies that can enhance patient outcomes.
What is an Investigational New Drug (IND) application?
An IND application is a formal submission to the U.S. Food and Drug Administration (FDA) seeking approval to initiate trials for a new drug or biologic in human subjects.
Why is the IND application important in drug development?
The IND application is crucial as it ensures that proposed studies are safe and scientifically valid, safeguarding the rights and welfare of trial participants.
What must an IND request demonstrate to be approved?
An IND request must convincingly demonstrate that the drug is reasonably safe for initial human use, supported by data from preclinical studies, including animal studies, pharmacology, and toxicology information.
What are the key components of an IND submission?
Key components include the IND Cover Letter, a comprehensive IND Submission Template, and an IND Protocol Template, which outline the clinical trial design, Chemistry, Manufacturing, and Controls (CMC) strategy, and any preliminary efficacy data.
What additional document might be required in an IND submission?
A Letter of Authorization (LOA) may be required for referencing existing IND information.
How has the number of IND requests changed recently?
In 2025, there was a notable rise in the number of IND requests submitted, indicating a growing interest in drug development.
What is the current approval rate for IND submissions?
The approval rates for IND submissions have remained robust, with a significant percentage receiving favorable outcomes.
What guidance does the FDA provide regarding the IND submission process?
The FDA has released guidance documents outlining the IND submission process, emphasizing adherence to regulatory guidelines while allowing flexibility in the submission approach.
Why is it important for sponsors to stay updated on regulatory expectations?
It is crucial for sponsors to remain aware of evolving regulatory expectations due to updates in the IND submission process, such as those made by the FDA in 2025, to navigate the complexities of drug development effectively.