


This article delves into the critical understanding of the interchangeability of biosimilars—biological products that closely resemble already approved biologics and can be substituted without the need for prescriber intervention. It underscores the rigorous regulatory frameworks, scientific evaluations, and clinical implications that underpin the safety and efficacy of biosimilars. Moreover, it addresses significant challenges, including:
Through this exploration, the article highlights the vital role biosimilars play in enhancing treatment options within the clinical landscape.
Understanding the intricacies of biosimilars is essential in today’s healthcare landscape, where these biological products are increasingly recognized for their potential to enhance patient access to effective treatments. As biosimilars gain traction, the concept of interchangeability—where a biosimilar can be substituted for its reference product without prescriber intervention—emerges as a pivotal focus for healthcare providers and patients alike. However, the journey toward embracing biosimilars is fraught with challenges. Stakeholders must navigate the complexities of regulatory frameworks and clinical implications to fully leverage the benefits of these innovative therapies.
How can we ensure that the transition to biosimilars is as seamless as possible for all involved?
Biosimilars are biological products that exhibit a high degree of similarity to already approved biologics, demonstrating no clinically meaningful differences in safety, purity, or potency. The interchangeability of biosimilars refers to the ability of a biosimilar to be substituted for its reference product without requiring the prescriber's intervention. This capability empowers pharmacists to replace the reference biologic with an interchangeable biosimilar at the point of dispensing, relying on the interchangeability of biosimilars to achieve the same clinical effect.
Understanding these definitions is crucial for navigating the regulatory landscape and recognizing the clinical implications of similar biological products. Recent data indicates that approximately 30% of individuals in the UK are utilizing similar biological products, reflecting a growing confidence in their safety and effectiveness. Real-world applications, such as the use of similar biologic products in managing autoimmune diseases, have demonstrated their efficacy, further solidifying their role in enhancing patient access to biologic therapies.
Expert opinions suggest that the increasing adoption of similar biological products is a positive trend, as they provide cost-effective alternatives to original biologics while maintaining comparable therapeutic outcomes.

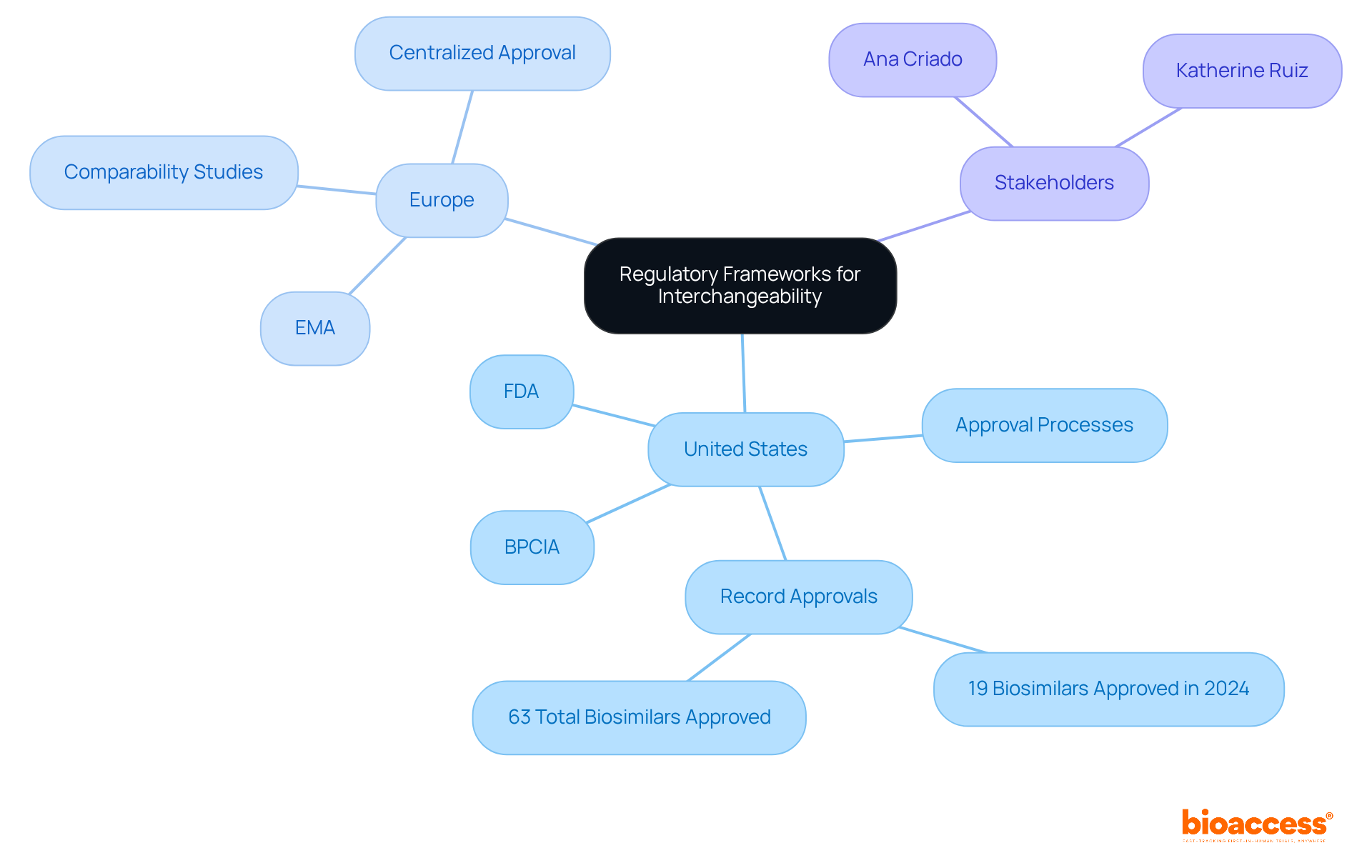
The regulatory frameworks governing biosimilars differ by region, yet they consistently uphold a commitment to rigorous evaluation processes that ensure reliability and efficacy. In the United States, the FDA has delineated a definitive pathway for biosimilar approval under the Biologics Price Competition and Innovation Act (BPCIA). To achieve the interchangeability of biosimilars, a biosimilar must demonstrate that it delivers the same clinical outcome as the reference product for any individual. This stipulation underscores the FDA's dedication to maintaining high standards for individual well-being and treatment effectiveness. In 2024, the FDA granted approval for a record 19 new biologics, signifying a substantial initiative to enhance market competition and patient access to affordable medications.
In a similar vein, the European Medicines Agency (EMA) has formulated guidelines that emphasize the importance of comprehensive comparability studies. These studies are crucial for confirming that a biosimilar is highly similar to its reference product, with no clinically meaningful differences in safety, purity, or potency. The EMA's centralized approval process facilitates access to biological medicines across EU member states, nurturing a competitive market environment.
Grasping these regulatory frameworks is essential for stakeholders in the biosimilar market, as they define the conditions under which the interchangeability of biosimilars can be established for marketing and utilization. Experts such as Ana Criado, with her extensive experience in regulatory affairs and biomedical engineering, and Katherine Ruiz, who specializes in regulatory affairs for medical devices and in vitro diagnostics, play pivotal roles in navigating these intricate regulations. As the biosimilars landscape continues to evolve, sustained dialogue and collaboration among regulatory bodies, manufacturers, and healthcare providers will be vital to ensure that biosimilars effectively address patient needs while promoting innovation within the pharmaceutical industry.
The principle of comparability underpins the interchangeability of biosimilars, necessitating thorough analytical and clinical evaluations to confirm that a biosimilar closely resembles its original product. This rigorous process encompasses a detailed assessment of both structural and functional characteristics, alongside clinical trials designed to evaluate safety and efficacy.
Recent studies underscore that alternating between similar biological products and their original counterparts does not introduce additional risk factors. Meta-analyses reveal no significant differences in major outcome measures, such as fatalities, serious adverse events, and treatment terminations. For instance, a systematic review involving 5,252 individuals indicated that the incidence of antidrug antibodies remained low, at less than 5%, suggesting that switching does not adversely affect immunogenicity. Furthermore, over 81% of participants exhibited no radiographic progression throughout the studies, bolstering the risk profile of similar biological products.
The totality of evidence approach is frequently employed, integrating data from various studies to substantiate claims of the interchangeability of biosimilars. This understanding is crucial for healthcare providers as they assess treatment alternatives and make informed decisions regarding patient care, particularly in light of the growing evidence supporting the safety and effectiveness of similar biological products.

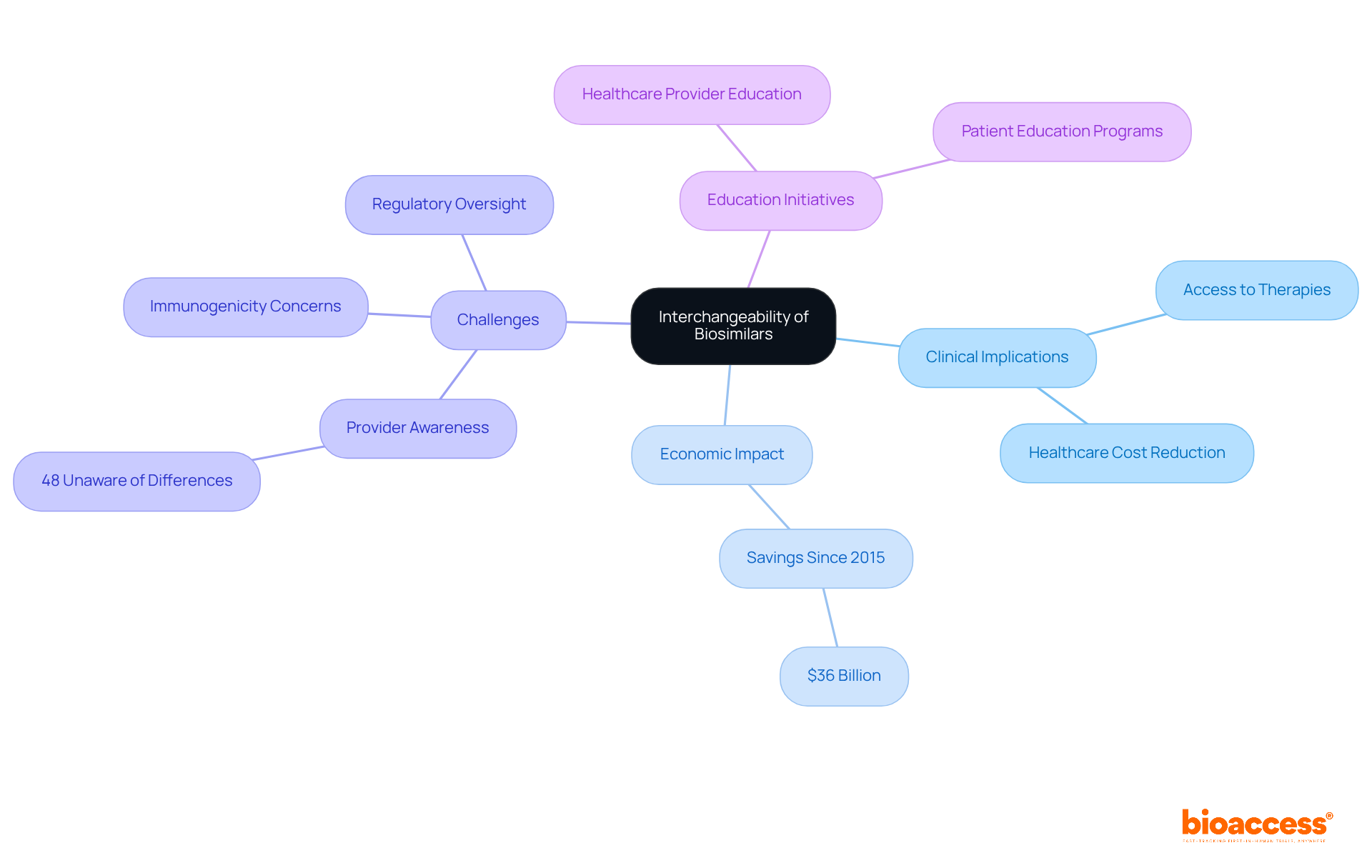
The clinical implications of the interchangeability of biosimilars are profound, as they significantly enhance access to biologic therapies while contributing to reduced healthcare costs. Since their inception, similar biologics have been utilized in nearly 700 million days of therapy, showcasing their efficacy without distinct clinical challenges. Furthermore, these biological products have generated $36 billion in savings since their market entry in 2015, underscoring their economic impact. However, challenges persist, particularly in educating healthcare providers and patients about the safety and efficacy of these alternatives. For instance, a systematic review indicated that 48% of healthcare professionals were unaware that similar biological products differ from generic medications, highlighting the urgent need for targeted educational initiatives.
Concerns about immunogenicity and the potential for non-medical switching further complicate the clinical landscape. A retrospective analysis revealed that anti-drug antibodies (ADAs) against reference biologics could cross-react with similar therapeutic agents, indicating the necessity for careful monitoring and documentation in pharmacovigilance. Additionally, a study discovered that 84% of doctors preferred not to transition stable individuals to similar medications without medical justifications, reflecting a reluctance based on safety and effectiveness issues. The stringent regulatory oversight imposed on similar biologics serves as a crucial basis for their market approval and consumer trust.
To address these challenges, healthcare provider education programs centered on the regulatory framework and clinical evidence supporting similar biological products are essential. Nurse-led education and structured communication strategies can enhance individual confidence and adherence, ultimately leading to improved health outcomes. As the landscape of biosimilars continues to evolve, it is imperative for clinicians to remain informed about the latest evidence and guidelines concerning the interchangeability of biosimilars to effectively incorporate them into treatment regimens while ensuring patient safety and confidence.
The interchangeability of biosimilars signifies a monumental advancement in the healthcare landscape, enabling the substitution of these biological products for their reference counterparts without necessitating prescriber intervention. This capability not only enhances patient access to essential therapies but also promotes cost-effective healthcare solutions while upholding safety and efficacy standards.
Throughout this article, we have explored key insights, including the definitions and regulatory frameworks underpinning biosimilars, the scientific principles ensuring their comparability, and the clinical implications of their use. The evidence presented underscores the successful integration of biosimilars in managing various health conditions, alongside the economic benefits they offer. However, challenges persist, particularly regarding the education and awareness of healthcare providers and patients concerning the safety and effectiveness of biosimilars.
As the biosimilars market continues to evolve, it is imperative for stakeholders—healthcare providers, regulatory bodies, and patients—to engage in ongoing education and dialogue. By fostering a deeper understanding of biosimilars and their interchangeability, the healthcare community can ensure that these innovative therapies are utilized effectively, ultimately improving patient outcomes and enhancing the overall healthcare system. Embracing the potential of biosimilars is not merely a choice; it is a necessary step toward a more accessible and sustainable future in medicine.
What are biosimilars?
Biosimilars are biological products that exhibit a high degree of similarity to already approved biologics, showing no clinically meaningful differences in safety, purity, or potency.
What does interchangeability mean in the context of biosimilars?
Interchangeability refers to the ability of a biosimilar to be substituted for its reference product without requiring the prescriber's intervention, allowing pharmacists to replace the reference biologic with an interchangeable biosimilar at the point of dispensing.
Why is understanding biosimilars and interchangeability important?
Understanding these definitions is crucial for navigating the regulatory landscape and recognizing the clinical implications of similar biological products.
What percentage of individuals in the UK are utilizing similar biological products?
Approximately 30% of individuals in the UK are utilizing similar biological products, indicating a growing confidence in their safety and effectiveness.
How are biosimilars used in real-world applications?
Biosimilars have been effectively used in managing autoimmune diseases, demonstrating their efficacy and enhancing patient access to biologic therapies.
What do experts think about the adoption of similar biological products?
Experts suggest that the increasing adoption of similar biological products is a positive trend, as they provide cost-effective alternatives to original biologics while maintaining comparable therapeutic outcomes.