


The investigator's brochure (IB) serves as a pivotal document that consolidates essential information regarding investigational products. This ensures that researchers and stakeholders grasp the product's safety and efficacy, which is vital for conducting ethical trials. A well-organized IB not only facilitates informed consent but also enhances transparency and compliance in clinical research. Consequently, this improvement leads to greater participant safety and bolsters the integrity of the study.
The investigator's brochure is a cornerstone in clinical research, serving as a comprehensive document that encapsulates critical information about investigational products. Its significance transcends mere compliance; it is vital for ensuring participant safety, fostering informed consent, and maintaining ethical standards throughout the research process. As the complexity of medical studies escalates, researchers must consider:
This article delves into the definition, importance, and key components of the investigator's brochure, shedding light on its pivotal role in enhancing transparency and integrity in clinical trials.
The investigator's brochure definition describes it as a pivotal document that consolidates both medical and non-medical information regarding a research product (IP) or trial medication. Its primary purpose is to furnish researchers and stakeholders with vital data concerning the product's safety, efficacy, and the investigator's brochure definition related to its use in research studies.
By acting as a foundational resource, the IB ensures that all individuals involved in a study are well-informed about the investigational product, in accordance with the investigator's brochure definition, which is essential for facilitating informed consent and maintaining ethical standards in trial conduct. A meticulously organized IB, as per the investigator's brochure definition, not only encapsulates available data but also plays a significant role in mitigating risks and bolstering the integrity of the research process.
Recent discussions have highlighted the necessity for clear communication within the IB, as statistics indicate that only 54% of participants fully grasp research objectives, underscoring the imperative for effective documentation. Furthermore, real-world examples demonstrate that comprehensive IBs can markedly improve informed consent rates, ultimately fostering trust and transparency in research.
As the landscape of medical studies evolves, it is vital to maintain a current and accessible IB to ensure participant safety and compliance with regulations. Regular updates to the investigator's brochure definition are crucial to reflect changes in the study product's safety profile and the latest research findings.
In this context, bioaccess's extensive trial management services—including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting—are essential to supporting the development and upkeep of effective IBs. Each of these services enhances the IB's efficacy by ensuring that the information is accurate, comprehensive, and communicated clearly, thereby improving participant comprehension and retention.
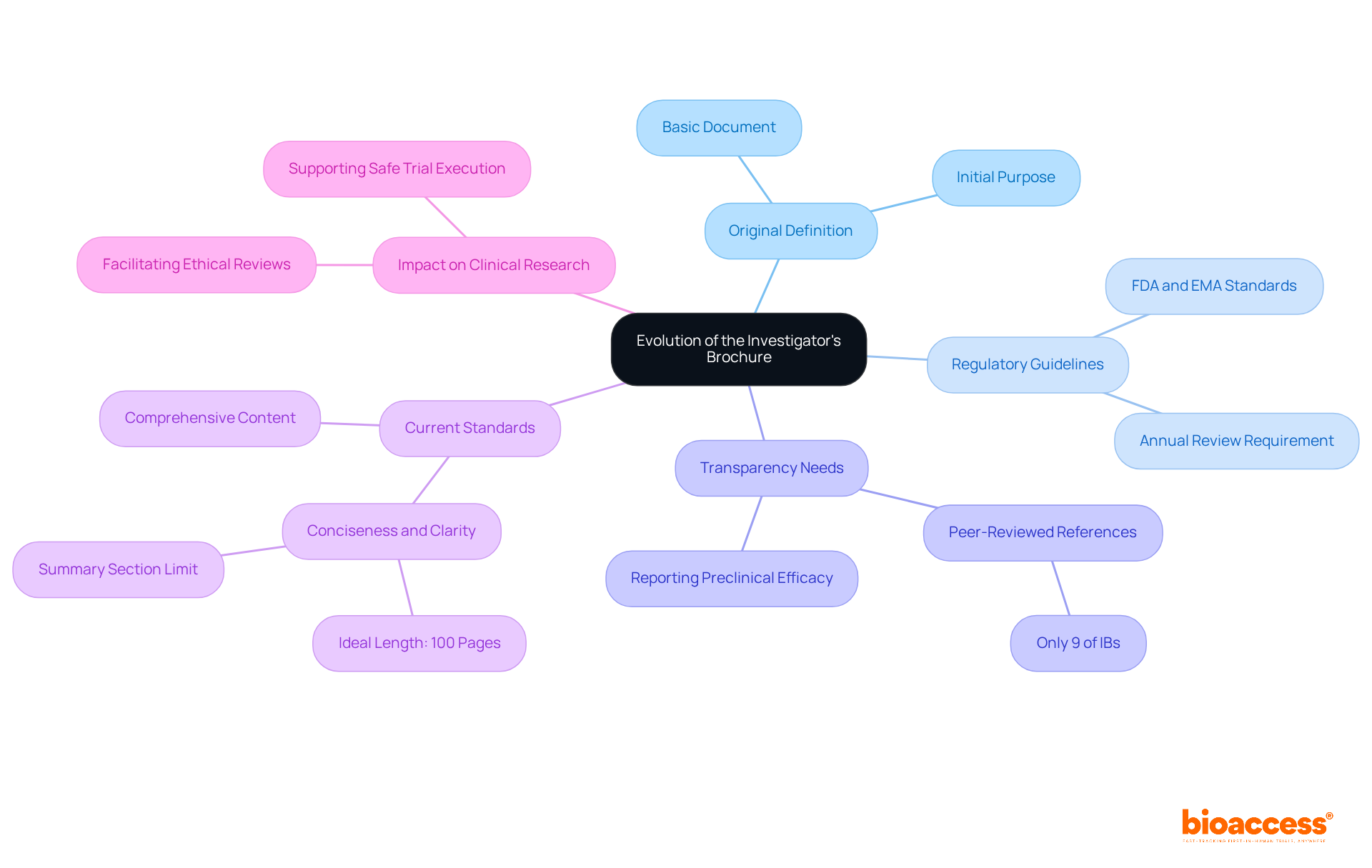
The investigator's brochure definition has undergone substantial evolution since its inception, marking a significant shift in its role within clinical research. Originally, the IB functioned as a straightforward document containing basic information about investigational products. However, as medical research has grown increasingly complex and regulatory scrutiny has intensified, the definition of the investigator's brochure has transformed into a comprehensive, multidisciplinary document.
Regulatory agencies, including the FDA and EMA, have established detailed guidelines that delineate essential components of the investigator's brochure definition, emphasizing the necessity for thorough data on safety, efficacy, and risk management. For example, a study analyzing 109 IBs revealed that only 9% referenced peer-reviewed publications, highlighting the critical need for improved transparency in reporting preclinical efficacy studies.
This evolution reflects a broader trend towards enhancing openness and informed decision-making in research studies, ensuring that investigators have access to the most pertinent information, which aligns with the investigator's brochure definition. Over the years, the IB has adapted to incorporate more rigorous standards, focusing on maintaining concise yet comprehensive content, ideally not exceeding 100 pages.
As regulatory requirements continue to evolve, the definition of the investigator's brochure remains an indispensable instrument for facilitating ethical review processes and supporting the safe execution of trials.
An investigator's brochure definition highlights its role as a vital document that encompasses several essential components to ensure comprehensive communication and compliance in clinical trials. The title page establishes the foundation of the document, containing the product's name, formulation, and sponsor details. Following this, the table of contents offers an organized overview, guiding readers through the document's sections and enhancing navigability.
The summary of clinical and non-clinical data presents pharmacological, toxicological, and pharmacokinetic information, providing a comprehensive understanding of the product's profile. The study protocol outlines the objectives, design, and methodology of the clinical trial, crucial for ensuring clarity in the research approach. Safety information is paramount, detailing known risks and adverse effects related to the experimental product. Recent research indicates that approximately 80% of delays or shutdowns arise from inadequate safety information in the IB, underscoring its significance. Furthermore, 60% of researchers express confidence in their understanding of the investigational brochure, highlighting the need for effective training programs.
Informed consent information offers guidance on effectively conveying risks to research participants, ensuring ethical standards are maintained. The references section provides a comprehensive list of relevant literature and studies that support the information presented in the IB, reinforcing its credibility. Collectively, these components ensure that the IB serves as a comprehensive resource for investigators, facilitating informed decision-making and ethical study conduct.
The thorough development of the IB is essential for the success of clinical studies. Notably, Phase III completion rates rise to 66% when local compliance knowledge is applied. As Sarah Lee states, 'The investigator's brochure definition is an essential document that describes the details of the investigational product, including its composition, pharmacology, toxicology, and research data.' By adhering to these guidelines, researchers can enhance the clarity and effectiveness of their Investigator's Brochures, ultimately contributing to improved participant safety and study integrity. Regular updates to the IB are also crucial to maintain its relevance and accuracy, ensuring that it reflects the latest findings and compliance requirements.

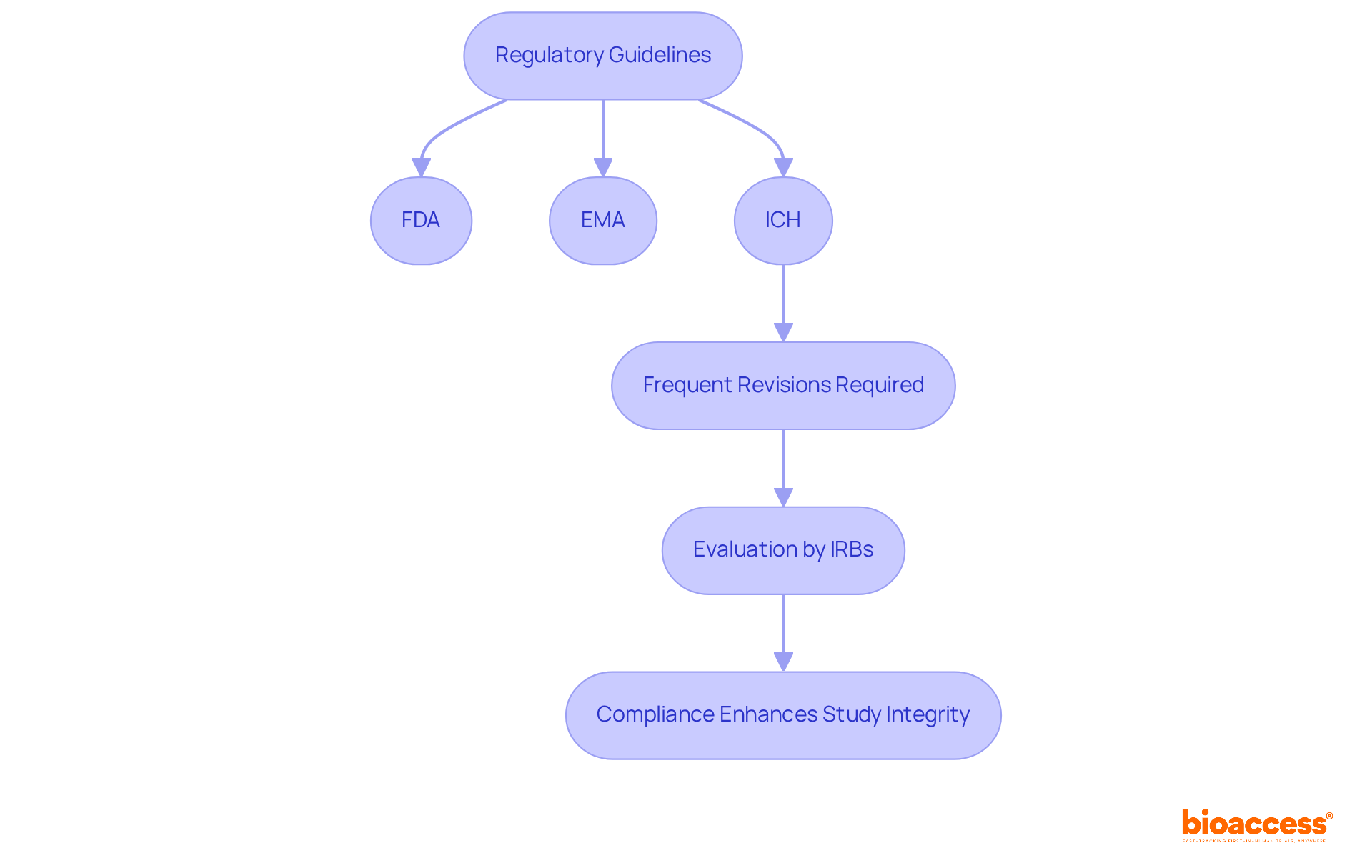
Regulatory guidelines for the investigator's brochure definition are set by key authorities such as the FDA, EMA, and ICH, ensuring that the content and structure of the IB meet rigorous standards for safety and efficacy reporting. The ICH E6 (R2) guidelines, for instance, mandate frequent revisions of the IB to incorporate new information and discoveries, reflecting the evolving landscape of medical research.
Furthermore, the IB must undergo evaluation and authorization by Institutional Review Boards (IRBs) prior to the initiation of research studies. Adhering to these regulations is essential, as it safeguards the rights and safety of participants while bolstering the credibility of the research.
A survey revealed that 55% of participants rated the content quality of IBs as satisfactory; however, areas for improvement in readability and comprehensibility were identified, particularly in sections critical for risk assessment.
By complying with these guidelines, sponsors and investigators can conduct their studies ethically and in alignment with best practices, ultimately enhancing the integrity of clinical trials.
The investigator's brochure is an essential document in clinical research, encapsulating critical information about investigational products and their implications for research studies. By providing a thorough overview of safety, efficacy, and ethical considerations, the IB equips all stakeholders to make informed decisions, thereby enhancing the integrity of clinical trials.
Throughout the article, the evolution of the investigator's brochure is highlighted, transitioning from a basic informational tool to a detailed, regulatory-compliant document. Clear communication, adherence to regulatory guidelines, and the inclusion of vital components such as safety information and informed consent processes are emphasized as fundamental to fostering trust and transparency in research. Additionally, the necessity for regular updates to the IB is underscored, reflecting ongoing advancements in medical knowledge and regulatory standards.
Given the pivotal role that the investigator's brochure plays in safeguarding participant rights and ensuring ethical study conduct, it is imperative for researchers and sponsors to prioritize the development and maintenance of effective IBs. By committing to comprehensive training and adherence to best practices, the research community can significantly enhance participant safety and the overall success of clinical trials. Embracing these principles not only bolsters the credibility of individual studies but also contributes to the advancement of medical research as a whole.
What is the investigator's brochure (IB)?
The investigator's brochure is a pivotal document that consolidates both medical and non-medical information regarding a research product or trial medication, providing vital data about its safety and efficacy.
What is the primary purpose of the investigator's brochure?
The primary purpose of the investigator's brochure is to furnish researchers and stakeholders with essential information about the investigational product, thereby facilitating informed consent and maintaining ethical standards in trial conduct.
Why is clear communication important in the investigator's brochure?
Clear communication is important because statistics indicate that only 54% of participants fully understand research objectives. Effective documentation in the IB can significantly improve informed consent rates and foster trust and transparency in research.
How does a well-organized investigator's brochure contribute to research integrity?
A meticulously organized investigator's brochure encapsulates available data, mitigates risks, and bolsters the integrity of the research process by ensuring that all individuals involved are well-informed about the investigational product.
Why is it important to keep the investigator's brochure updated?
It is vital to maintain a current and accessible investigator's brochure to ensure participant safety and compliance with regulations, reflecting changes in the study product's safety profile and the latest research findings.
What services does bioaccess provide to support the development of effective investigator's brochures?
Bioaccess offers extensive trial management services, including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting, all of which enhance the efficacy of the investigator's brochure by ensuring accurate and comprehensive information is communicated clearly.