


The article emphasizes the critical importance of understanding antibody isotypes, particularly their structures and functions, which are essential for immune responses and therapeutic applications. It delineates the unique characteristics of each isotype—illustrating IgG's pivotal role in long-term immunity and IgA's significant function in mucosal defense. These distinctions are not merely academic; they inform the selection of antibodies for both research and clinical purposes, ultimately enhancing treatment strategies and improving patient outcomes. This comprehensive overview serves as a foundation for further exploration into the implications of antibody diversity in clinical research.
Understanding the intricacies of antibody isotypes is essential for anyone delving into immunology. These unique classes—IgG, IgA, IgM, IgE, and IgD—play pivotal roles in the immune response. Each isotype possesses distinct structural and functional characteristics that dictate their interactions with pathogens, influencing therapeutic strategies and research methodologies. Given this diversity, how can researchers and clinicians effectively navigate the complexities of antibody isotypes? Optimizing treatment outcomes and enhancing the efficacy of studies hinges on this understanding.
The isotype of antibody, also known as immunoglobulin classes, is classified into five primary types: IgG, IgA, IgM, IgE, and IgD. Each isotype of antibody exhibits unique structural and functional characteristics that dictate its role in the immune system. For instance,
Understanding these differences is crucial for researchers and clinicians, as they greatly affect the choice of the isotype of antibody for therapeutic applications and diagnostic purposes. Recent studies emphasize the significance of antibody types in creating targeted therapies, with monoclonal antibodies being utilized in managing various conditions, including autoimmune diseases and cancers. Ongoing research into the mechanisms and applications of these variants continues to drive innovations in immunotherapy and diagnostics.
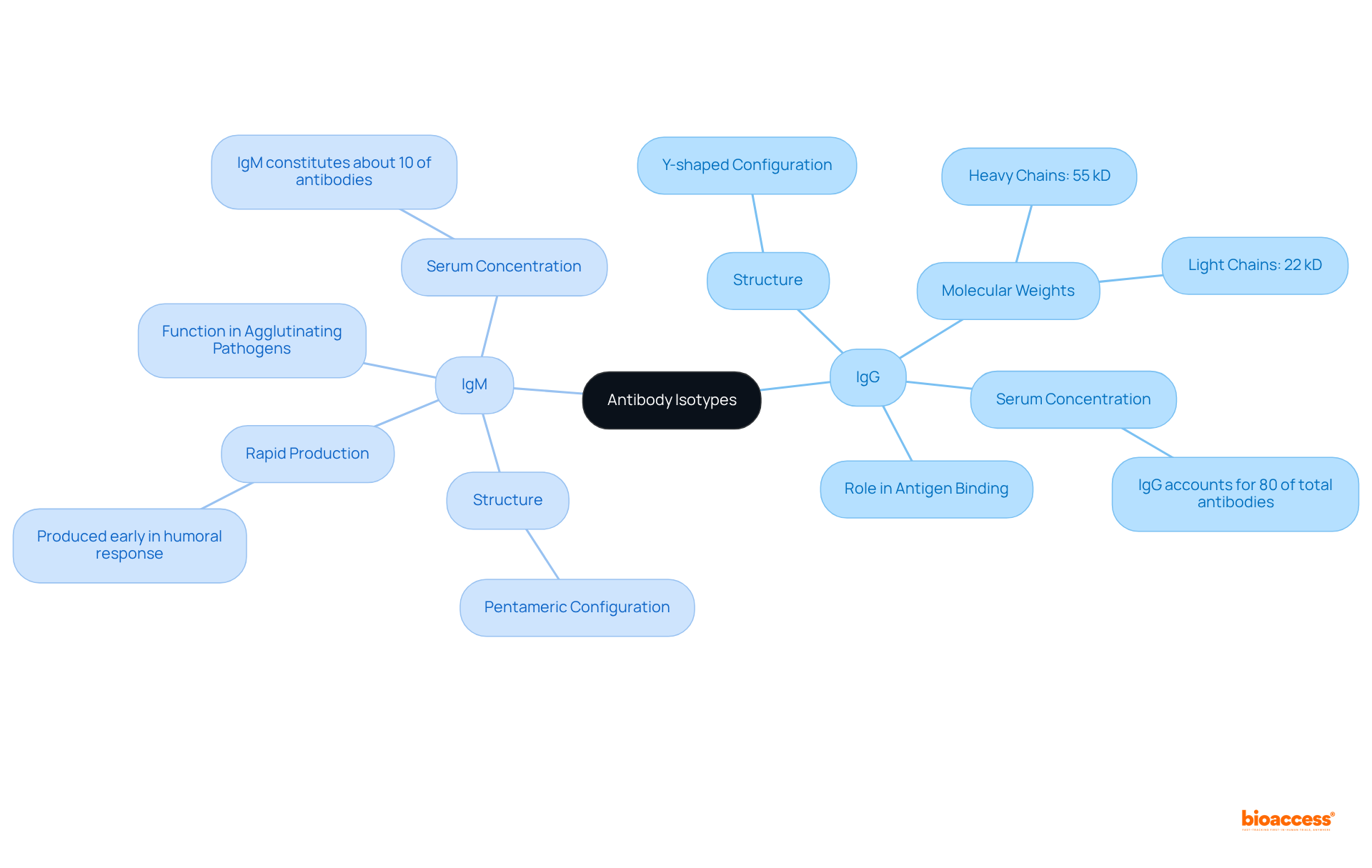
Distinct structural characteristics are exhibited by the isotype of antibody, which are pivotal to its functional roles in the immune system. For instance, IgG features a Y-shaped configuration composed of two heavy chains (each with a molecular weight of 55 kD) and two light chains (each with a molecular weight of 22 kD), facilitating effective antigen binding. In contrast, IgM is structured as a pentamer, comprising five interconnected Y-shaped units, significantly enhancing its capacity to agglutinate pathogens and activate complement through the classical pathway. Furthermore, IgM is produced rapidly early in the humoral response, providing immediate protection.
The distinctive constant regions found in the heavy chains of each variant, which represent an isotype of antibody, determine their interactions with diverse immune cells and complement proteins, affecting their effector functions. Recent studies have highlighted the significance of these structural characteristics, revealing that IgG comprises approximately 80% of the total immunoglobulins in circulation, while IgM accounts for about 10%. Comprehending these distinctions is vital for choosing the suitable isotype of antibody for particular therapeutic uses and research efforts, as these isotypes can also function as diagnostic indicators for different illnesses.
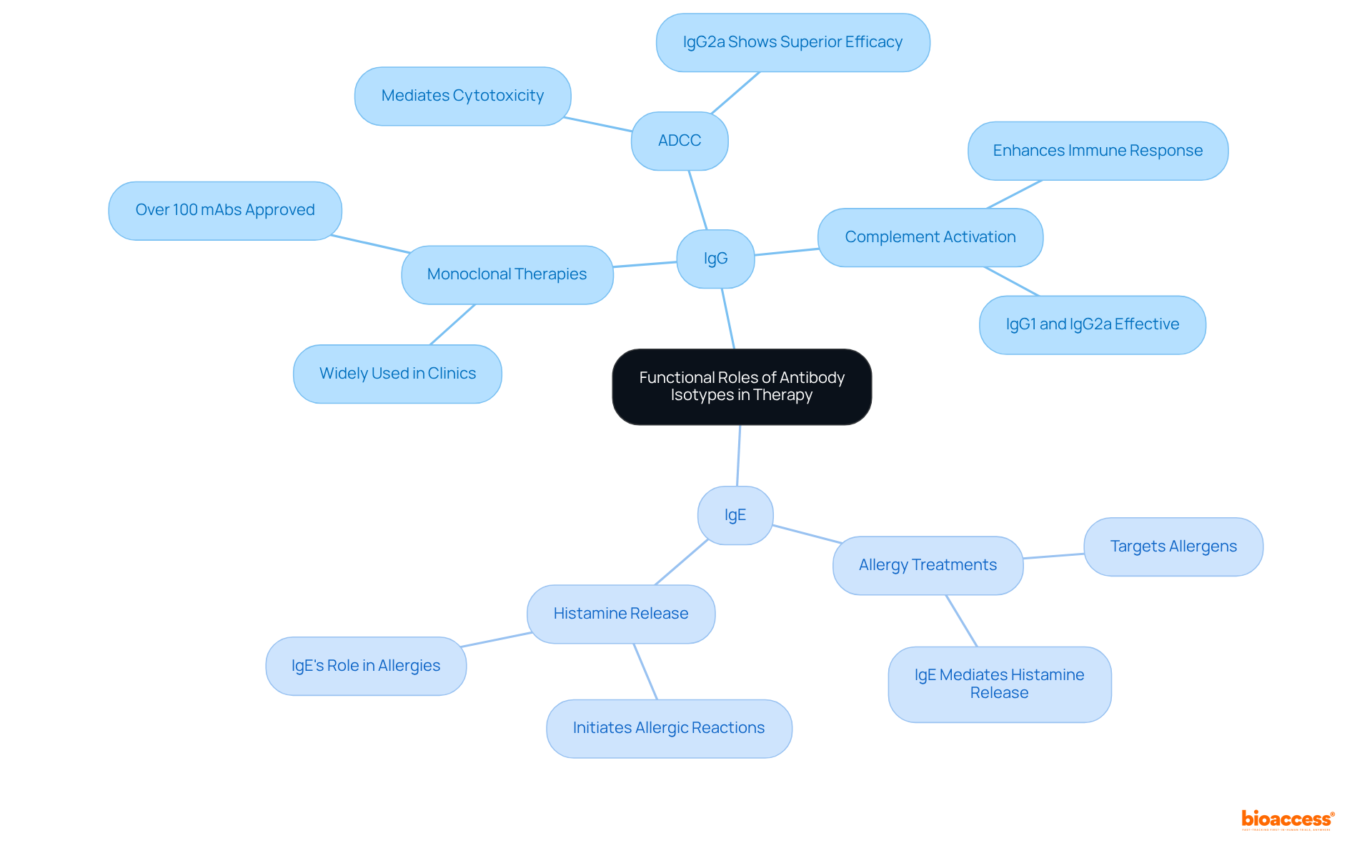
The role of the isotype of antibody is crucial in therapeutic applications. For instance, IgG is widely employed in monoclonal therapies due to its ability to mediate antibody-dependent cellular cytotoxicity (ADCC) and activate complement pathways. In contrast, IgE is pivotal in allergy treatments, as it binds to allergens and initiates histamine release from mast cells. Understanding the functional characteristics of each isotype of antibody empowers researchers and clinicians to tailor their approaches in immunotherapy, vaccine development, and disease management, ultimately ensuring optimal patient outcomes.
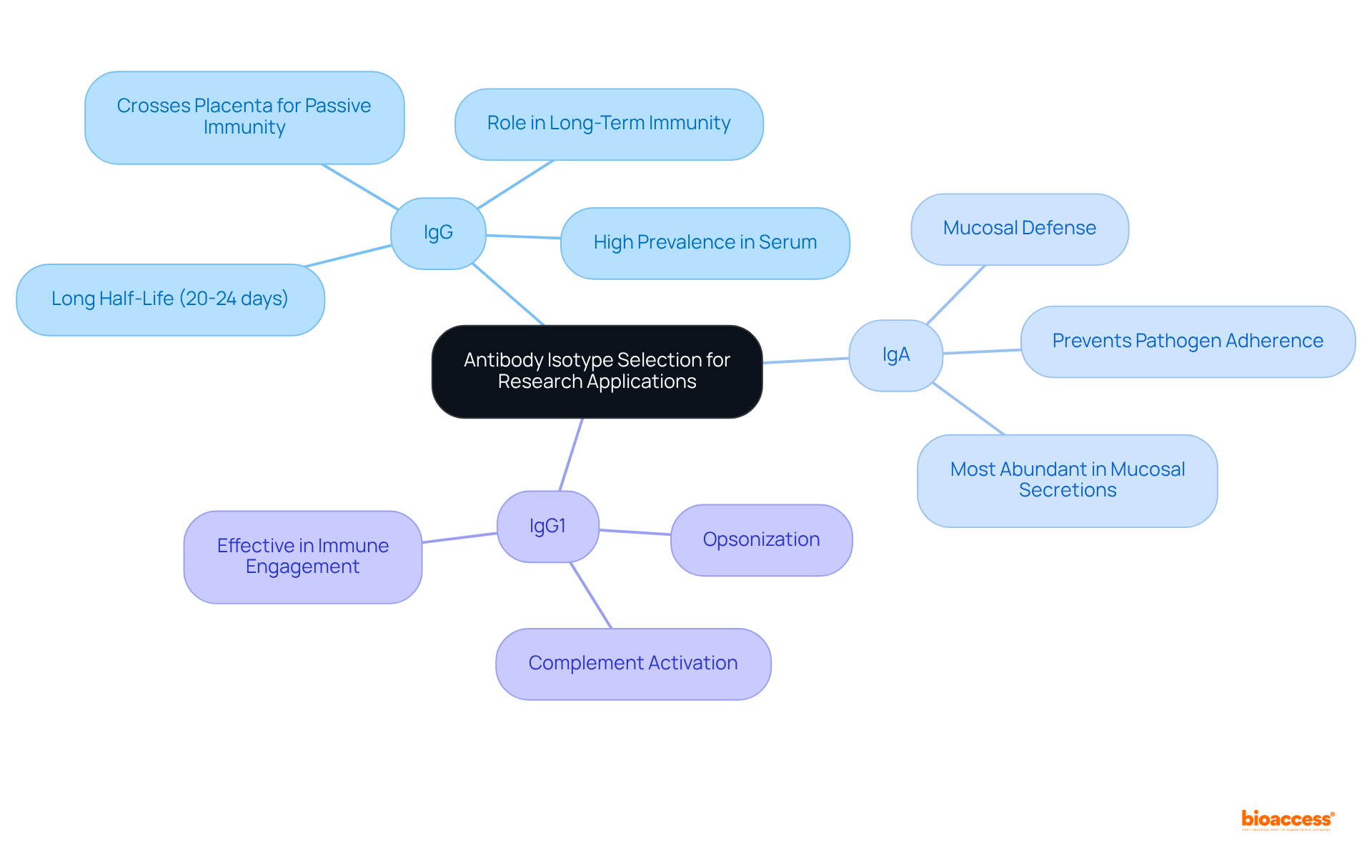
Selecting the appropriate isotype of antibody is crucial for the success of research applications, particularly when considering the specific objectives of your study. For example, IgG is frequently the preferred choice for investigating immune responses due to its high prevalence and functionality in serum, accounting for approximately 70-85% of total immunoglobulins. Its long half-life of 20-24 days further supports its role in providing sustained immunity, and it possesses the unique ability to cross the placenta, offering passive immunity to the fetus. Conversely, if your research targets mucosal immunity, IgA is more appropriate, as it plays a vital role in preventing pathogen adherence at mucosal surfaces and is the most abundantly produced immunoglobulin in humans, representing about 5-15% of the antibody pool.
Moreover, the capacity of a specific isotype of antibody to activate complement or engage immune cells can significantly influence experimental outcomes. For instance, IgG1 is particularly effective in opsonization and complement activation, making it ideal for studies that require robust immune engagement. In contrast, while IgA is less efficient in complement activation, it is indispensable for mucosal defense, underscoring the necessity of aligning your selection of isotype with your research goals.
Expert recommendations emphasize that researchers should thoroughly evaluate their study objectives alongside the distinct functions of each isotype of antibody. This careful consideration can enhance the validity and relevance of findings, ultimately leading to more impactful research outcomes. Understanding the nuances of antibody isotypes not only aids in selecting the right tools for experiments but also contributes to a broader comprehension of immune responses in various contexts.
Understanding the isotype of antibodies is essential in the field of immunology. These unique classes—IgG, IgA, IgM, IgE, and IgD—each play distinct roles that are crucial for effective immune responses. Their varied structures and functions dictate how they interact with pathogens and influence therapeutic strategies and research applications. The significance of selecting the appropriate antibody isotype cannot be overstated; it can determine the success of treatment and the validity of experimental outcomes.
This article has explored the unique structural features of each antibody isotype and their corresponding functional roles.
Furthermore, the importance of these distinctions in therapeutic applications, such as monoclonal antibody therapies and vaccine development, has been highlighted, emphasizing the need for tailored approaches based on the specific isotype's characteristics.
In summary, a comprehensive understanding of antibody isotypes is imperative for both researchers and clinicians. This knowledge enhances the ability to select the right isotype for therapeutic and research purposes and contributes to advancements in immunotherapy and diagnostics. Embracing the nuances of antibody isotypes can lead to more effective treatments and a deeper understanding of immune responses, ultimately improving patient care and outcomes.
What are the main types of antibody isotypes?
The five primary types of antibody isotypes are IgG, IgA, IgM, IgE, and IgD.
What role does IgG play in the immune system?
IgG constitutes about 80% of total immunoglobulins and is crucial for opsonization and neutralization of pathogens, making it vital for long-term immunity.
Where is IgA primarily found, and what is its function?
IgA makes up approximately 15% of all immune proteins and is primarily found in mucosal regions, acting as a barrier against pathogens that enter through mucosal surfaces.
What is unique about IgM compared to other antibody isotypes?
IgM is the largest immunoglobulin, primarily existing as a pentamer, which enhances its ability to bind multiple antigens and is the first antibody produced in response to an infection.
What role does IgE play in the immune response?
Although present in small amounts in the bloodstream, IgE plays a significant role in allergic reactions and provides defense against parasitic infections.
What is the function of IgD, and where is it located?
IgD is located mainly on B-lymphocytes and represents about 0.25% of immunoglobulins in the human body. It plays a role in B cell activation, although its exact functions are still being explored.
Why is understanding antibody isotypes important for researchers and clinicians?
Understanding the differences between antibody isotypes is crucial as they greatly affect the choice of antibody for therapeutic applications and diagnostic purposes.
How are monoclonal antibodies used in medicine?
Monoclonal antibodies are utilized in managing various conditions, including autoimmune diseases and cancers, highlighting the significance of different antibody types in creating targeted therapies.
What is the current focus of research regarding antibody isotypes?
Ongoing research focuses on the mechanisms and applications of antibody isotypes, driving innovations in immunotherapy and diagnostics.