


The article underscores the critical importance of medical devices, systematically categorizing them by risk levels and highlighting their pivotal role in both clinical research and patient care. It establishes credibility by detailing the regulatory framework set forth by authorities such as INVIMA, which is instrumental in ensuring the safety and efficacy of these devices. Furthermore, it illustrates the historical evolution of medical devices and the integration of advanced technologies that significantly enhance healthcare outcomes.
The landscape of healthcare is intricately woven with the presence of medical devices, which play a pivotal role in enhancing patient care and clinical outcomes. These instruments, ranging from simple bandages to complex imaging technologies, are classified based on risk, ensuring they meet rigorous safety and effectiveness standards. As the medical device industry evolves with advancements in technology and regulatory frameworks, stakeholders face the challenge of navigating this complex terrain to leverage these tools for improved healthcare delivery. By exploring the classifications, historical context, and key components of medical devices, we uncover not only their significance but also the challenges and opportunities that lie ahead in modern medicine.
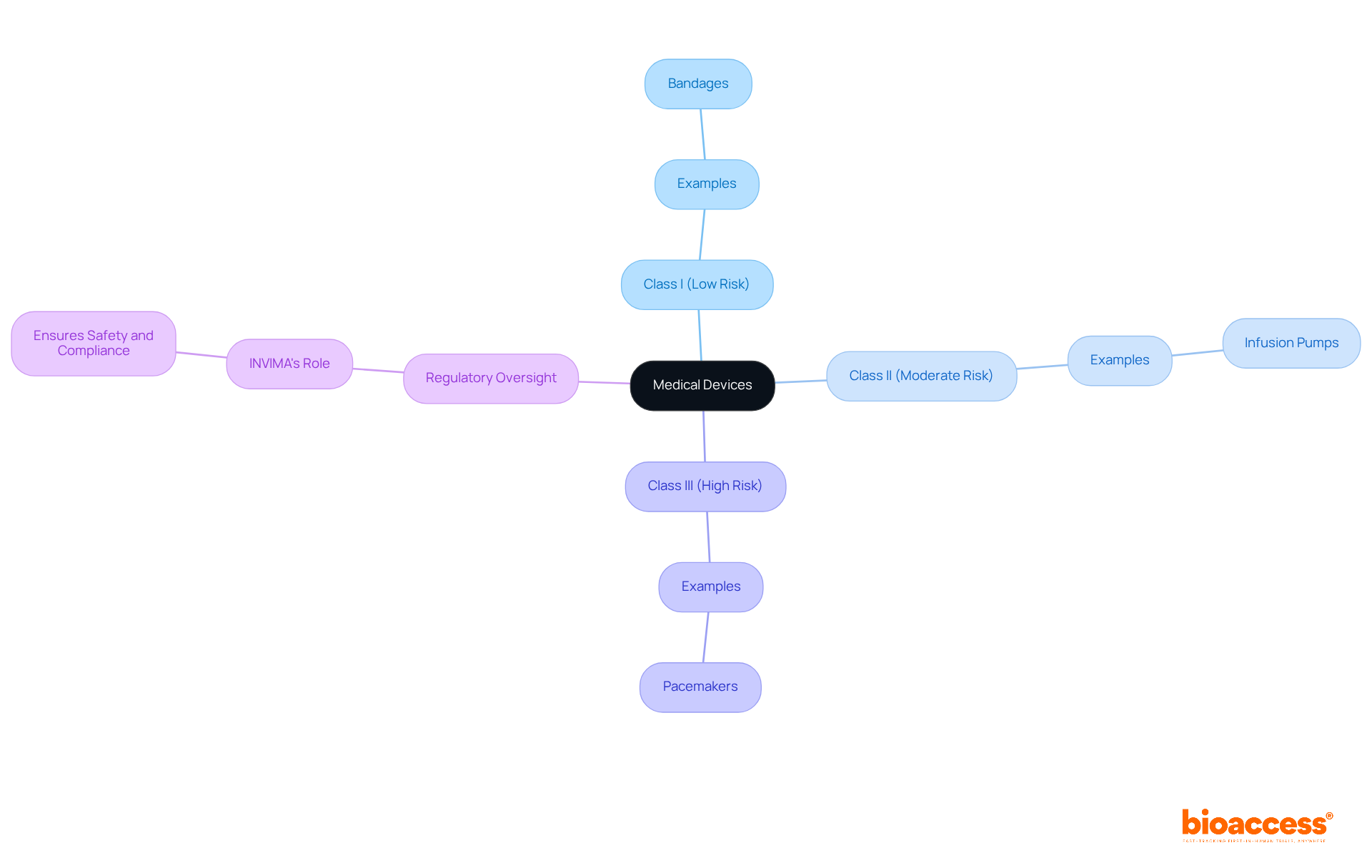
A list of medical devices encompasses a wide range of healthcare instruments designed for clinical purposes, including diagnosis, prevention, monitoring, treatment, and relief of illnesses. These instruments are categorized into three primary classes based on risk:
This classification is vital for regulatory compliance and ensures that products meet stringent safety and effectiveness standards before they enter the market. In Colombia, the oversight of healthcare instruments falls under the jurisdiction of INVIMA (Instituto Nacional de Vigilancia de Medicamentos y Alimentos), recognized as a Level 4 health authority by the Pan American Health Organization/World Health Organization. INVIMA is responsible for regulating healthcare instruments, guaranteeing adherence to established safety and quality standards.
Understanding these classifications and the regulatory landscape is essential for stakeholders navigating the complexities of the development and approval processes for the list of medical devices. Furthermore, companies like bioaccess® offer specialized services that facilitate expedited clinical trials for Medtech, Biopharma, and Radiopharma startups, ensuring a more efficient journey through the compliance maze.
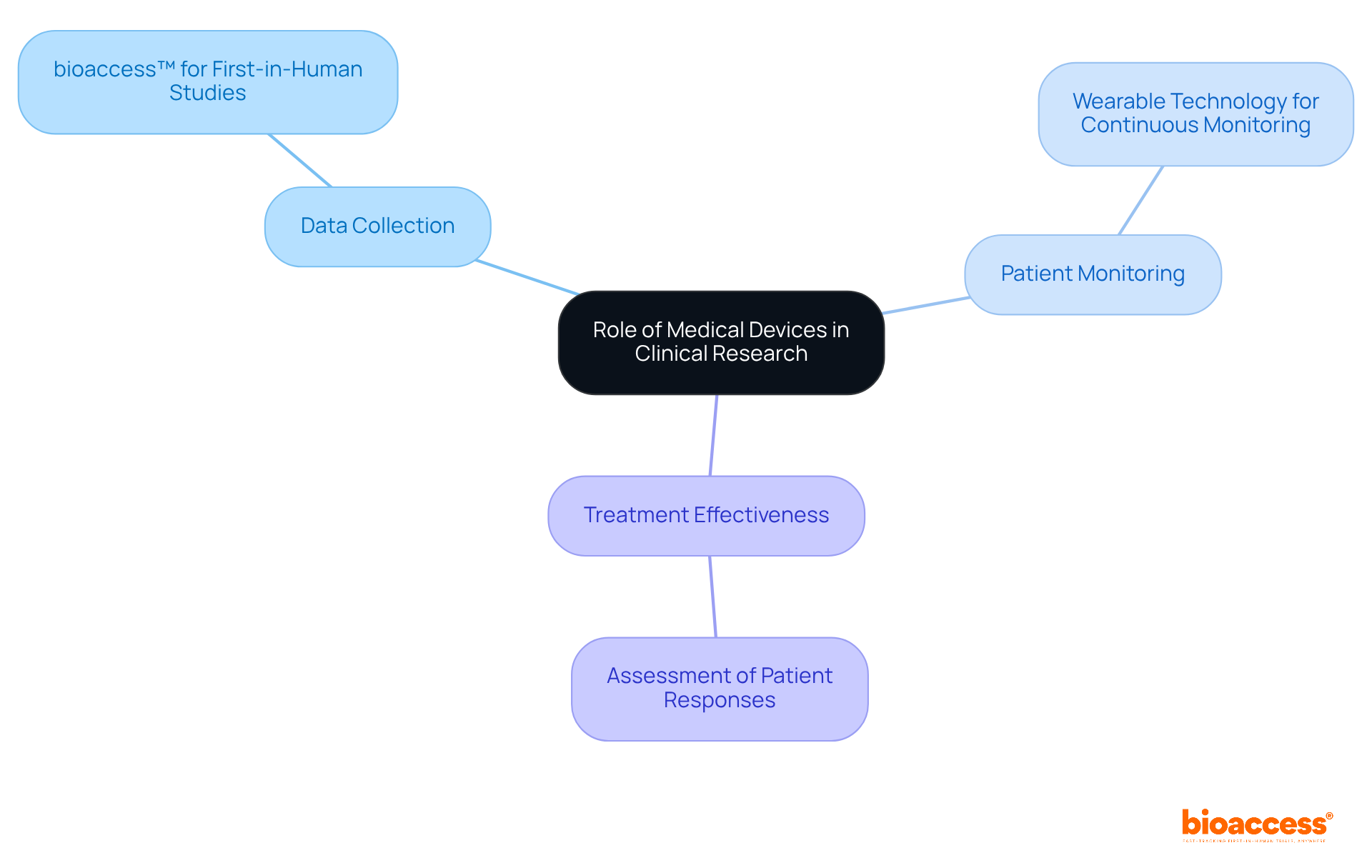
Medical instruments are indispensable in clinical research, acting as essential tools for data collection, patient monitoring, and treatment effectiveness assessment. They enable first-in-human studies and early feasibility trials, empowering researchers to evaluate safety and performance in real-world contexts.
For example, bioaccess™ aids Avantec Vascular in its first-in-human clinical study of an innovative vascular product in Latin America by facilitating the selection of a principal investigator and the submission of its regulatory dossier for ministry of health approvals.
Additionally, wearable technology enables continuous monitoring of vital signs, providing invaluable insights into patient responses during trials.
The integration of health instruments in clinical studies not only accelerates the development of groundbreaking treatments but also enhances the overall standard of care for patients, as illustrated by Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, during his initial human trial in Colombia.
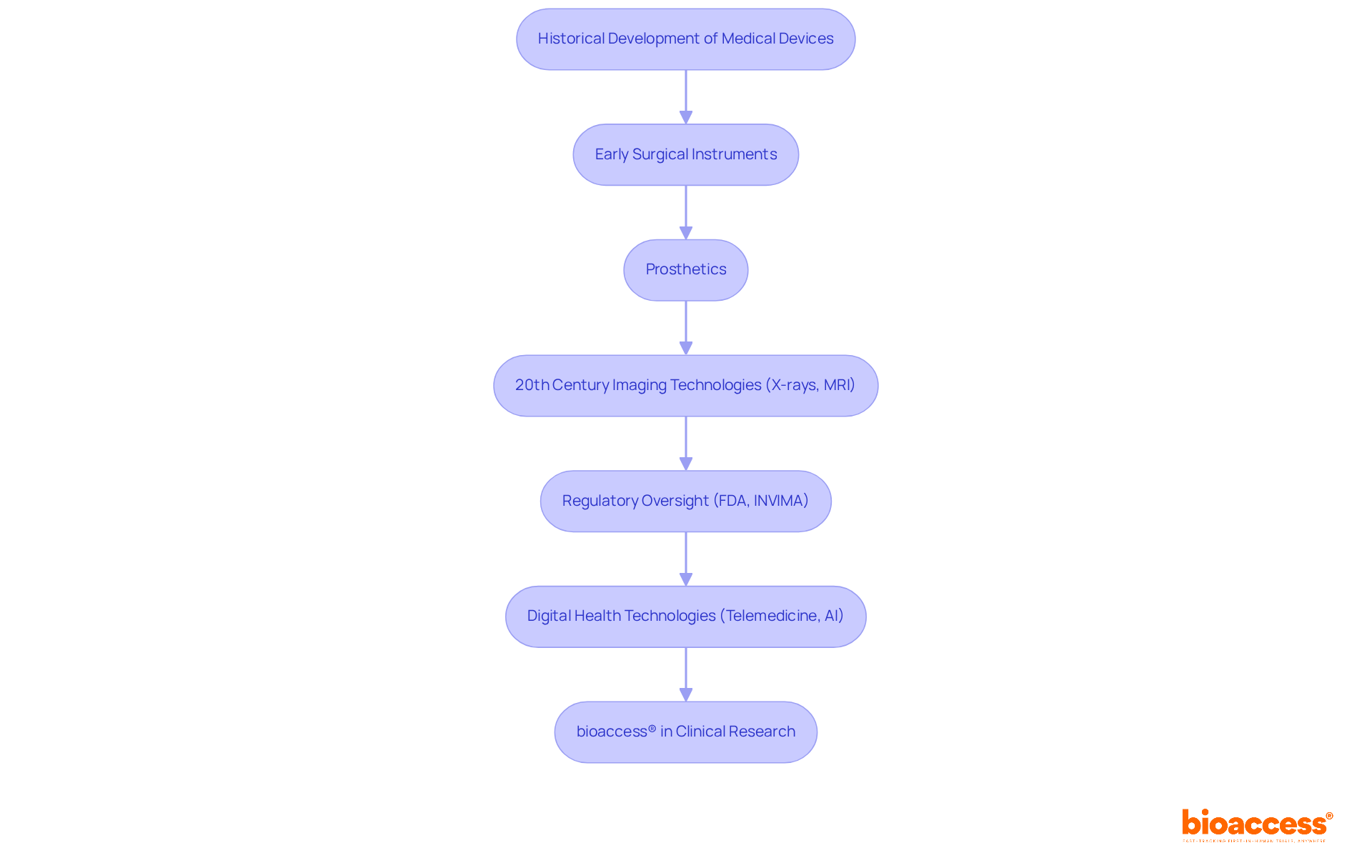
The development of healthcare tools has a rich history, dating back centuries with early innovations including surgical instruments and prosthetics. The 20th century heralded significant advancements, particularly with the advent of imaging technologies such as X-rays and MRI machines, which revolutionized diagnostics. Regulatory bodies, including the FDA in the United States and INVIMA (Colombia National Food and Drug Surveillance Institute), have played a crucial role in shaping the landscape by ensuring the safety and efficacy of a list of medical devices. Notably, INVIMA is recognized as a Level 4 medical authority by the Pan American Health Organization/World Health Organization, overseeing the marketing and manufacturing of wellness products in Colombia and ensuring compliance with safety standards.
Today, the integration of digital health technologies—such as telemedicine and AI-powered diagnostics—continues to transform the field, reflecting a trend towards more personalized and efficient healthcare solutions. In this evolving landscape, bioaccess® emerges as a leading contract research organization (CRO) in Latin America, facilitating accelerated clinical research for Medtech startups. With expertise across multiple clinical trial stages, including:
bioaccess® is dedicated to enhancing healthcare tools through customized solutions and specialized assistance.
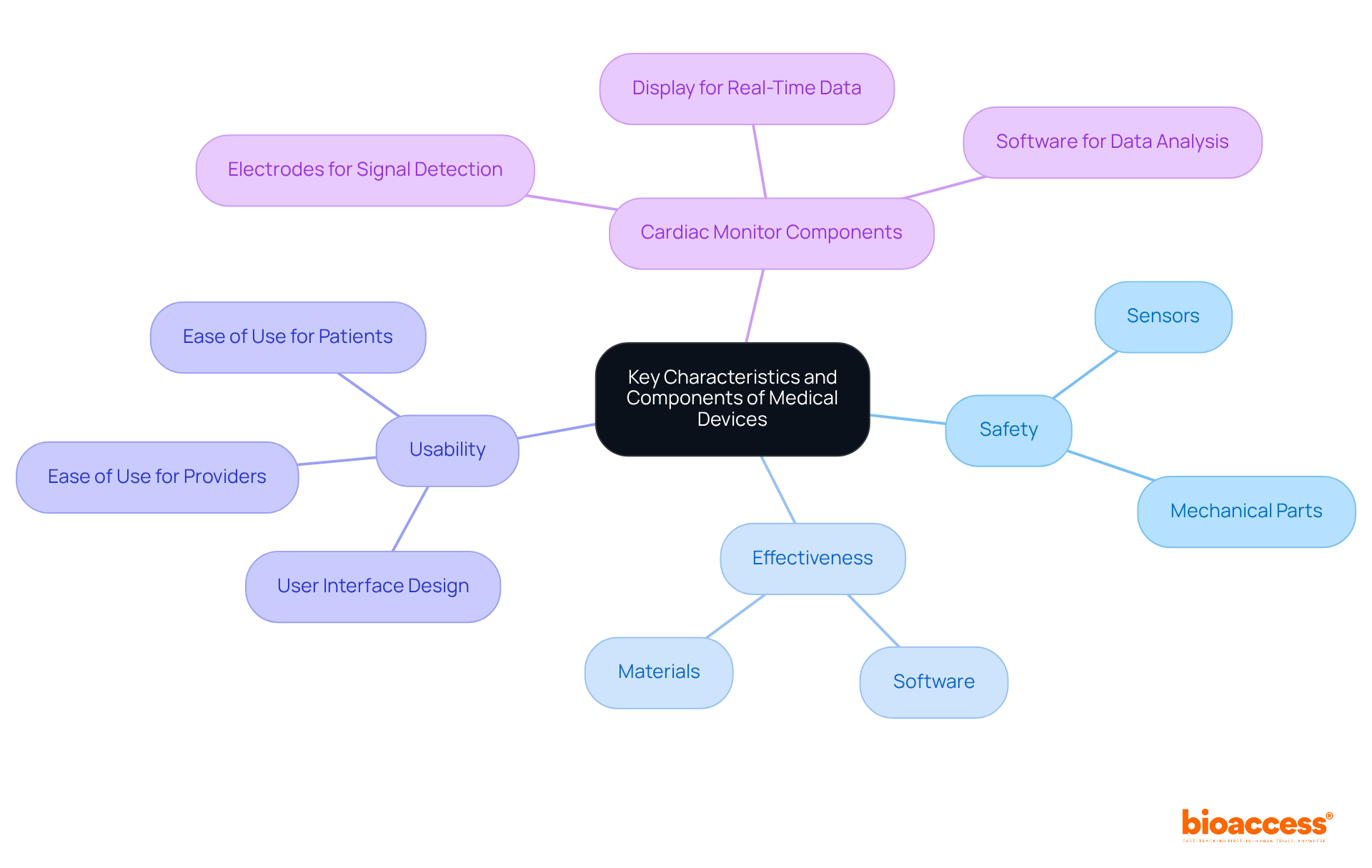
Key traits of healthcare instruments include safety, effectiveness, and usability. Safety ensures that instruments do not pose excessive danger to patients, while effectiveness relates to their ability to achieve desired wellness outcomes. Usability involves the design and user interface, which must facilitate ease of use for both healthcare providers and patients.
The components of the list of medical devices can vary widely, encompassing:
Each plays a crucial role in the device's overall performance. For instance, a cardiac monitor may incorporate:
All working in unison to deliver essential health information.
The significance of medical devices within the healthcare ecosystem cannot be overstated. These instruments play a pivotal role in diagnosing, monitoring, and treating patients, ensuring that care is both effective and safe. By categorizing medical devices into different risk classes, stakeholders can navigate regulatory frameworks more efficiently, ultimately leading to innovations that enhance patient outcomes.
Throughout the article, various aspects of medical devices have been explored, from their classification and regulatory oversight by entities like INVIMA to their indispensable role in clinical research. The evolution of these devices highlights a journey marked by technological advancements, from basic surgical tools to sophisticated digital health technologies. The integration of such innovations not only accelerates the development of new treatments but also raises the standard of care provided to patients.
In reflecting on the broader implications, it becomes clear that the future of healthcare is inextricably linked to the ongoing evolution of medical devices. As technology continues to advance, the potential for improved patient outcomes and enhanced healthcare delivery grows exponentially. Stakeholders in the medical field must remain committed to understanding and leveraging these advancements, ensuring that they contribute positively to the landscape of modern medicine.
What are medical devices?
Medical devices are healthcare instruments designed for clinical purposes, including diagnosis, prevention, monitoring, treatment, and relief of illnesses.
How are medical devices categorized?
Medical devices are categorized into three primary classes based on risk: Class I (low risk, such as bandages), Class II (moderate risk, like infusion pumps), and Class III (high risk, including pacemakers).
Why is the classification of medical devices important?
The classification is vital for regulatory compliance and ensures that products meet stringent safety and effectiveness standards before they enter the market.
Who regulates healthcare instruments in Colombia?
In Colombia, the regulation of healthcare instruments falls under the jurisdiction of INVIMA (Instituto Nacional de Vigilancia de Medicamentos y Alimentos), which is recognized as a Level 4 health authority by the Pan American Health Organization/World Health Organization.
What is INVIMA's role in the regulation of medical devices?
INVIMA is responsible for regulating healthcare instruments, ensuring adherence to established safety and quality standards.
Why is it important for stakeholders to understand medical device classifications and regulations?
Understanding these classifications and the regulatory landscape is essential for stakeholders navigating the complexities of the development and approval processes for medical devices.
What services do companies like bioaccess® provide?
Companies like bioaccess® offer specialized services that facilitate expedited clinical trials for Medtech, Biopharma, and Radiopharma startups, ensuring a more efficient journey through the compliance process.