


The role of a clinical research monitor is crucial in ensuring compliance with regulatory guidelines, protecting participant welfare, and maintaining the integrity of clinical trial data. Monitors conduct site inspections, verify data accuracy, and address challenges—essential tasks for the successful execution of research studies and the advancement of medical knowledge. Their expertise not only safeguards participants but also fortifies the reliability of the trial outcomes, which is vital for the progression of medical innovations. In this capacity, clinical research monitors serve as the backbone of clinical trials, ensuring that all processes adhere to the highest standards of ethics and scientific rigor.
The intricate world of clinical trials hinges on a multitude of factors, yet one role emerges as a linchpin in ensuring success: the clinical research monitor. These professionals transcend mere oversight; they are the guardians of ethical standards and data integrity, ensuring that every aspect of a study adheres to stringent regulations. Acting as the bridge between research sponsors and clinical sites, they play a critical role in safeguarding participant welfare while enhancing the credibility of research outcomes.
However, the path is fraught with challenges, from navigating complex regulatory landscapes to managing relationships under pressure. What truly defines the impact of a clinical research monitor, and how do they navigate these demanding responsibilities?
A Research Supervisor plays a vital role in managing research tests, ensuring compliance with regulatory guidelines, project protocols, and ethical standards. Their primary responsibility involves protecting the rights and welfare of participants while upholding the integrity of the data collected. This encompasses:
Acting as a crucial link between the sponsor and the research site, the monitor fosters communication and addresses any challenges that may arise during the study. By enforcing strict compliance with protocols and regulations, Research Monitors are essential to the successful execution of studies, making significant contributions to advancements in medical knowledge and patient care. Their role is further emphasized by the necessity for effective monitoring practices, which include:
These practices not only enhance participant safety but also ensure the credibility and reliability of research outcomes, ultimately fostering trust in the medical investigation process.

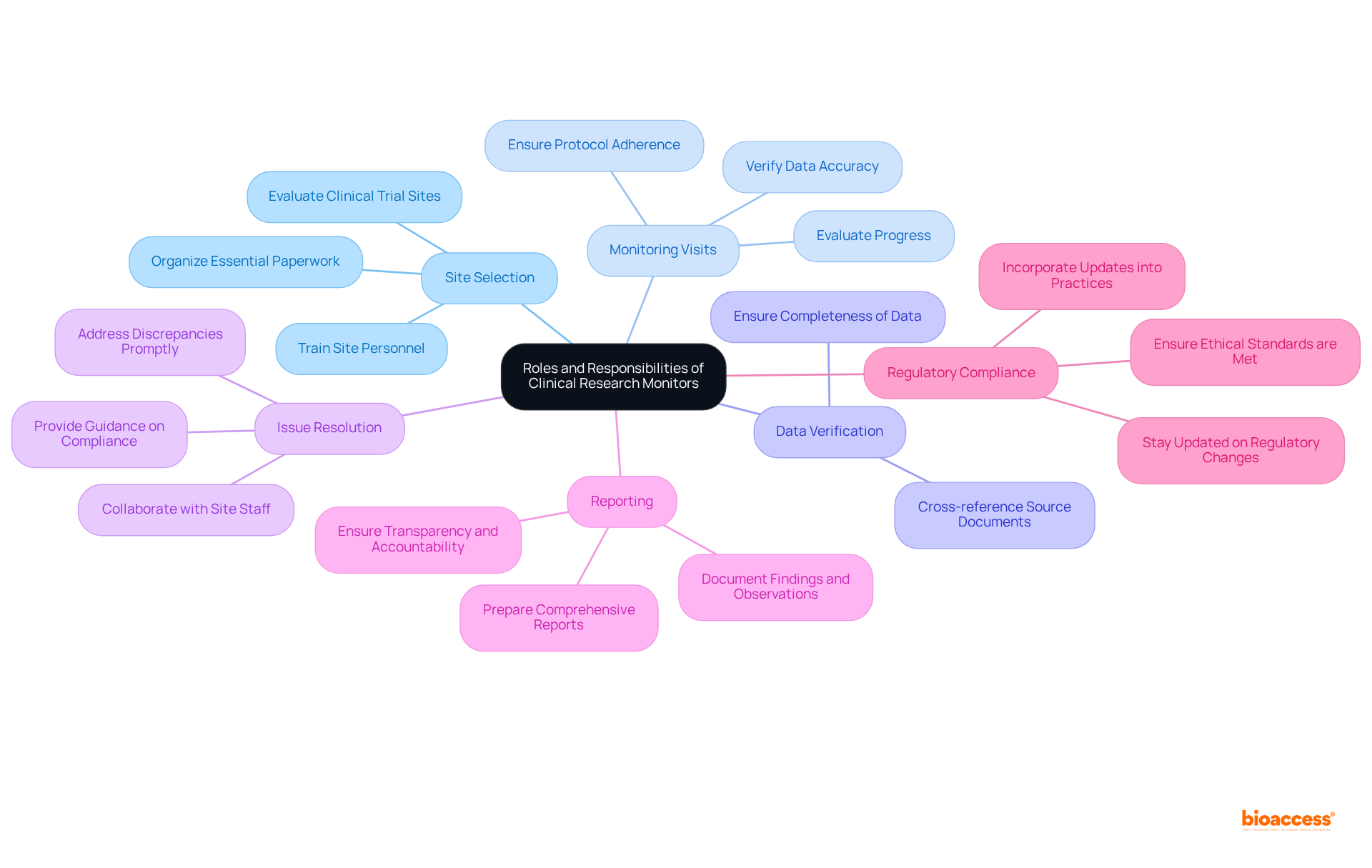
The roles and responsibilities of a clinical research monitor are vital for the effective management of clinical trials, encompassing a variety of essential tasks.
Monitors play a crucial role in site selection and initiation, evaluating clinical trial sites for their capabilities and resources. They ensure that all essential paperwork is organized and that site personnel receive adequate training to facilitate a seamless project initiation.
Regular monitoring visits are conducted to evaluate progress, verify data accuracy, and ensure adherence to the protocol. During these visits, monitors assess compliance with Good Clinical Practice (GCP) and regulatory requirements, which are fundamental for maintaining study integrity.
A key responsibility of the clinical research monitor is data verification, which involves cross-referencing source documents with case report forms (CRFs) to ensure the accuracy and completeness of data collected at the site. This meticulous attention to detail is crucial for the reliability of the research outcomes.
When discrepancies or issues arise, monitors collaborate with site staff to address them promptly, offering guidance on protocol compliance or participant safety concerns. This proactive approach ensures that the study remains on course and that participant rights are protected.
Following site visits, monitors prepare comprehensive reports documenting findings, observations, and any corrective actions taken. These documents are essential for ensuring transparency and accountability throughout the research process.
Ensuring compliance with all applicable regulations and ethical standards is a fundamental responsibility of a clinical research monitor. They must remain vigilant regarding regulatory changes and incorporate these updates into their monitoring practices to uphold the integrity of the study.
Through these duties, Research Monitors not only ensure that studies are conducted ethically and efficiently but also contribute significantly to the advancement of medical research and improved patient outcomes. Their role is crucial in enhancing the reliability of research findings, ultimately leading to better healthcare solutions.
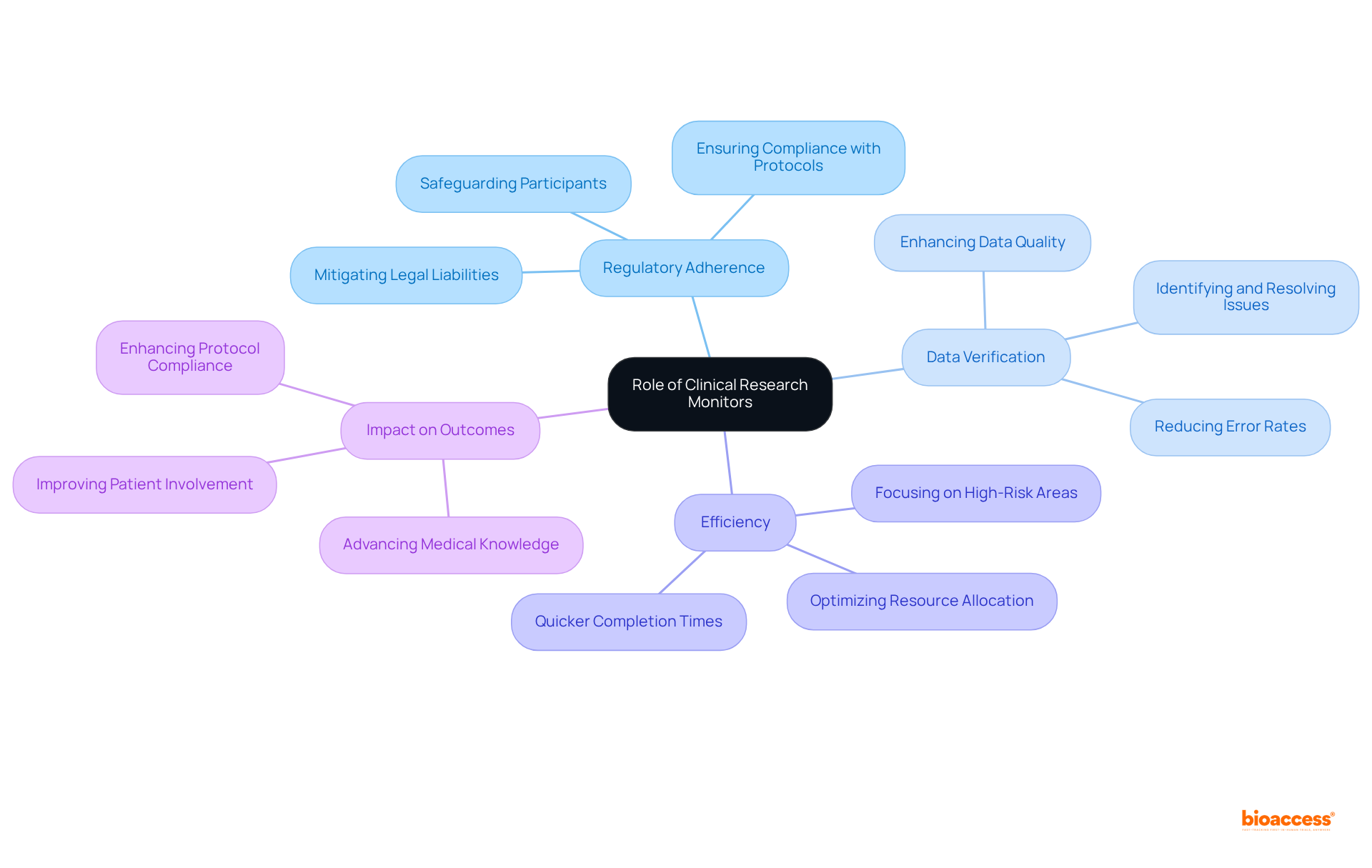
The role of clinical research monitors is pivotal to the success of research studies, as they ensure adherence to regulatory standards that are essential for the validity of study results. Their rigorous oversight not only safeguards the rights and welfare of participants but also fosters trust in the role of the clinical research monitor in the research process.
Furthermore, the clinical research monitor plays a critical role in data verification and issue resolution, significantly enhancing the quality of collected data, which is crucial for making informed decisions regarding the safety and efficacy of new treatments. Effective monitoring practices by a clinical research monitor have been shown to correlate with quicker completion times, as potential issues are identified and resolved promptly. This efficiency benefits sponsors and researchers alike while accelerating the availability of new therapies to patients in need.
With over 20 years of experience in Medtech, bioaccess® achieves enrollment 50% faster than conventional markets, underscoring the effectiveness that a clinical research monitor contributes to the study process. Additionally, bioaccess® emphasizes extensive research management services, including Early-Feasibility Studies, First-In-Human Studies, and Post-Market Follow-Up Studies, which are vital for advancing medical device research in Latin America.
Case studies demonstrate that skilled clinical research monitors help mitigate legal liabilities, enhance protocol compliance, and boost patient involvement, all of which improve study efficiency. Ultimately, the presence of adept clinical research monitors is essential for preserving the integrity and success of studies, advancing medical knowledge, and enhancing patient outcomes.
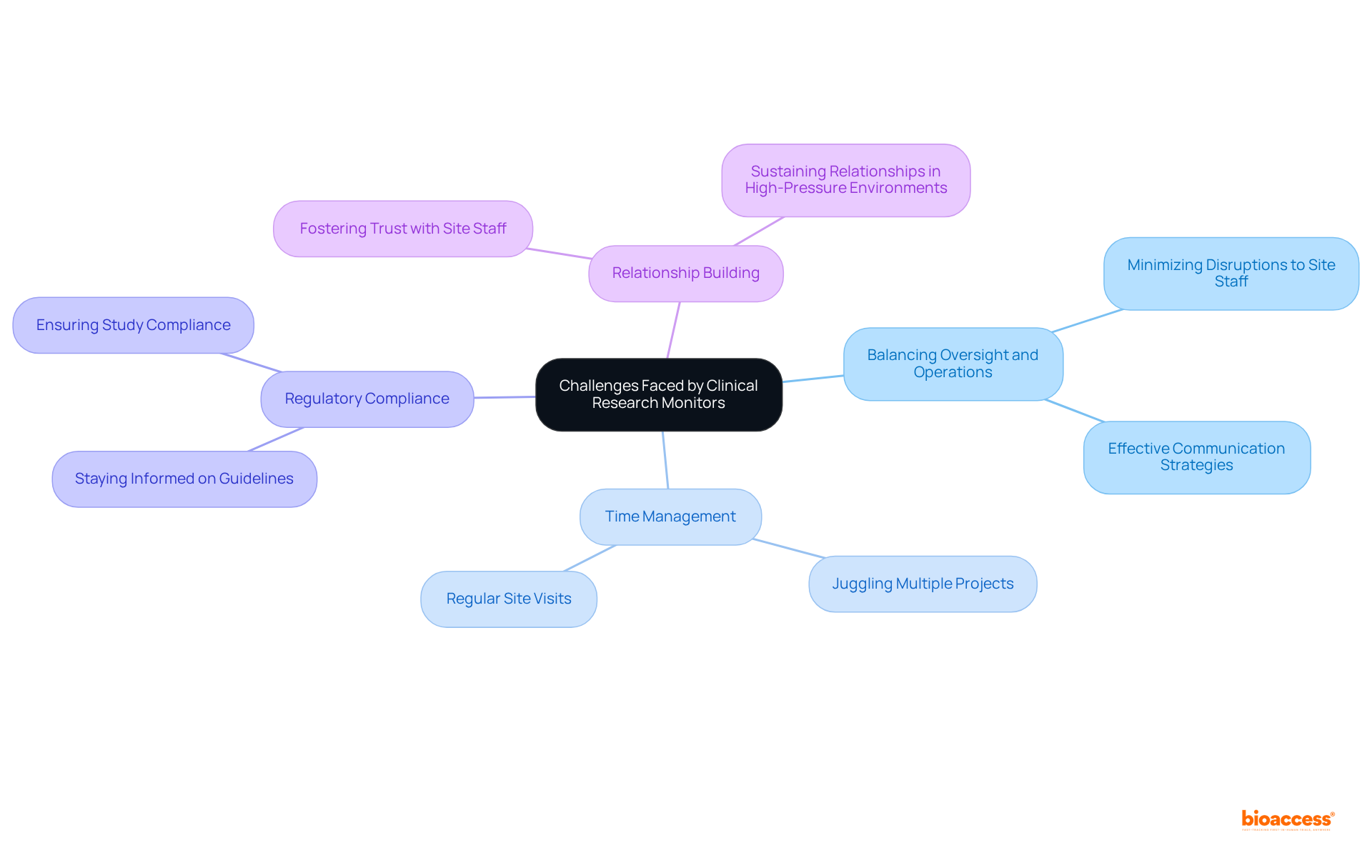
A clinical research monitor faces a range of challenges that significantly impact the effectiveness of clinical trials. A primary concern is the need to balance comprehensive oversight with the operational demands of clinical sites. Monitors must ensure compliance while minimizing disruptions to site staff workflows; this requires exceptional interpersonal skills and effective communication strategies.
Additionally, time management emerges as a vital concern; monitors often juggle multiple projects and must conduct regular site visits, complicating their ability to maintain consistent supervision across all locations. The evolving regulatory landscape introduces further complexity, as monitors must stay informed about changes in guidelines and ensure that all studies comply with the latest standards.
Furthermore, fostering and sustaining relationships with site staff and investigators can prove particularly challenging in high-pressure environments. By acknowledging these challenges, clinical research monitors can develop strategies to navigate them effectively, thereby enhancing the successful execution of clinical trials.
The role of a clinical research monitor is integral to the successful execution of clinical trials, as they ensure compliance with regulatory standards and uphold the ethical treatment of participants. By acting as a bridge between sponsors and research sites, monitors play a crucial part in safeguarding data integrity and participant welfare throughout the research process.
Key responsibilities include:
Their proactive approach to identifying and addressing issues not only enhances the quality of research outcomes but also fosters trust in the clinical trial process. The importance of effective monitoring practices is underscored by their ability to streamline studies, mitigate risks, and ultimately contribute to advancements in medical knowledge and patient care.
In light of the challenges faced by clinical research monitors, it is essential to recognize their vital contributions to the field. As the landscape of clinical research continues to evolve, the demand for skilled monitors will only increase. Embracing their role not only enhances the efficiency of trials but also plays a significant part in ensuring that new treatments are developed safely and effectively. Investing in robust monitoring practices is crucial for the future of medical research, ultimately leading to improved healthcare solutions for patients worldwide.
What is the role of a Clinical Research Monitor?
A Clinical Research Monitor is responsible for managing research tests, ensuring compliance with regulatory guidelines, project protocols, and ethical standards, while protecting the rights and welfare of participants and maintaining data integrity.
What are the primary responsibilities of a Research Monitor?
The primary responsibilities include conducting regular site inspections, reviewing research documentation, and ensuring adherence to Good Clinical Practice (GCP) guidelines.
How does a Research Monitor facilitate communication during a study?
The Research Monitor acts as a crucial link between the sponsor and the research site, fostering communication and addressing any challenges that may arise during the study.
Why is compliance with protocols and regulations important in clinical research?
Compliance with protocols and regulations is essential for the successful execution of studies, contributing significantly to advancements in medical knowledge and patient care.
What practices enhance the effectiveness of monitoring in clinical research?
Effective monitoring practices include meticulous planning, rigorous data verification, and ongoing training for personnel.
How do monitoring practices impact participant safety and research outcomes?
These practices enhance participant safety and ensure the credibility and reliability of research outcomes, ultimately fostering trust in the medical investigation process.