


The role of a clinical trial monitor is essential in ensuring compliance with regulations, safeguarding participant safety, and preserving data integrity throughout clinical studies. Monitors serve as pivotal facilitators of communication between sponsors and research sites, diligently overseeing adherence to protocols and upholding ethical standards.
Collectively, these responsibilities are vital for the successful execution of medical research, reflecting the critical importance of collaboration in the clinical research landscape.
The intricate world of clinical research hinges on the meticulous oversight provided by clinical trial monitors, also known as Clinical Research Associates (CRAs). These professionals ensure adherence to regulatory standards and ethical practices while safeguarding the integrity of the data collected, which is vital for the advancement of medical science. As the complexity of clinical trials increases, one must consider the specific skills and responsibilities essential for these monitors to navigate the challenges they face in their critical role.
A clinical trial monitor, also referred to as a Clinical Research Associate (CRA), plays an indispensable role in overseeing clinical studies. Their primary responsibility is to ensure that these studies adhere to regulatory requirements, research protocols, and Good Clinical Practice (GCP) guidelines. This oversight is vital for protecting the rights and well-being of participants while ensuring the integrity of the data collected. The clinical trial monitor acts as the principal communication link between the sponsor and the research site, facilitating seamless operations and swiftly addressing any issues that may arise.
The importance of clinical trial monitors extends beyond mere compliance; they are pivotal to the overall success of medical studies. Their expertise is crucial in identifying discrepancies in clinical data, which may signal potential fraud or errors. For example, a case study highlighted how effective monitoring practices led to the early detection of data inconsistencies, ultimately preserving the integrity of the experiment.
Furthermore, the role of a clinical trial monitor is significant in ensuring the ethical conduct of experiments. They guarantee that informed consent is secured from participants and that ethical standards are upheld throughout the study. This is especially critical in studies involving vulnerable populations, where the risks must be justified by potential benefits. Recent updates in regulatory frameworks underscore stringent ethical oversight, reinforcing the necessity of a clinical trial monitor in upholding high standards of participant safety.
In conclusion, the role of clinical trial monitors is essential for ensuring compliance, enhancing data integrity, and promoting ethical practices in medical investigations. Their contributions not only facilitate the smooth implementation of studies but also uphold the trust and safety of participants, ultimately leading to more reliable and impactful outcomes.
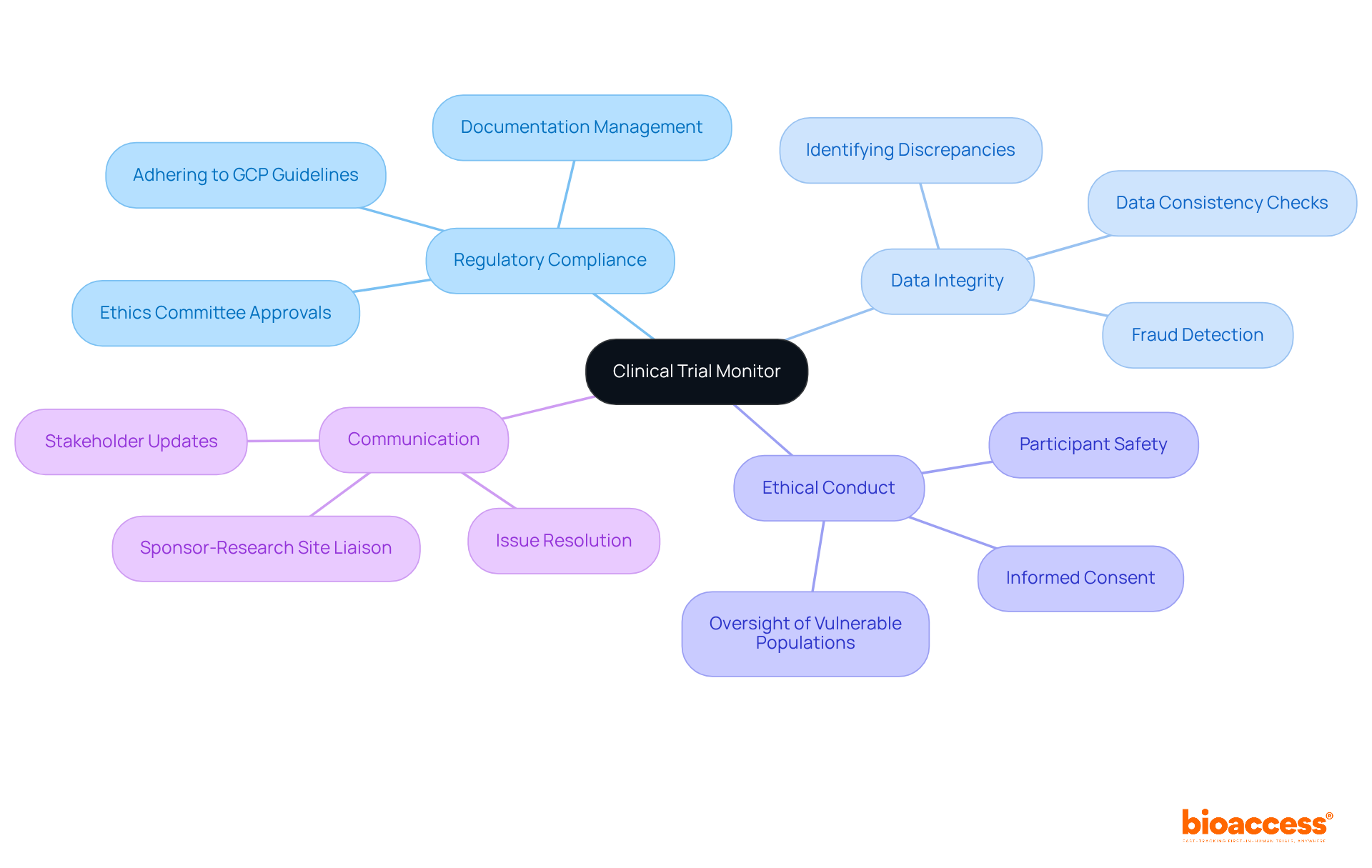
Clinical trial monitors play a crucial role in ensuring the integrity and compliance of clinical research, with a diverse range of responsibilities that include:
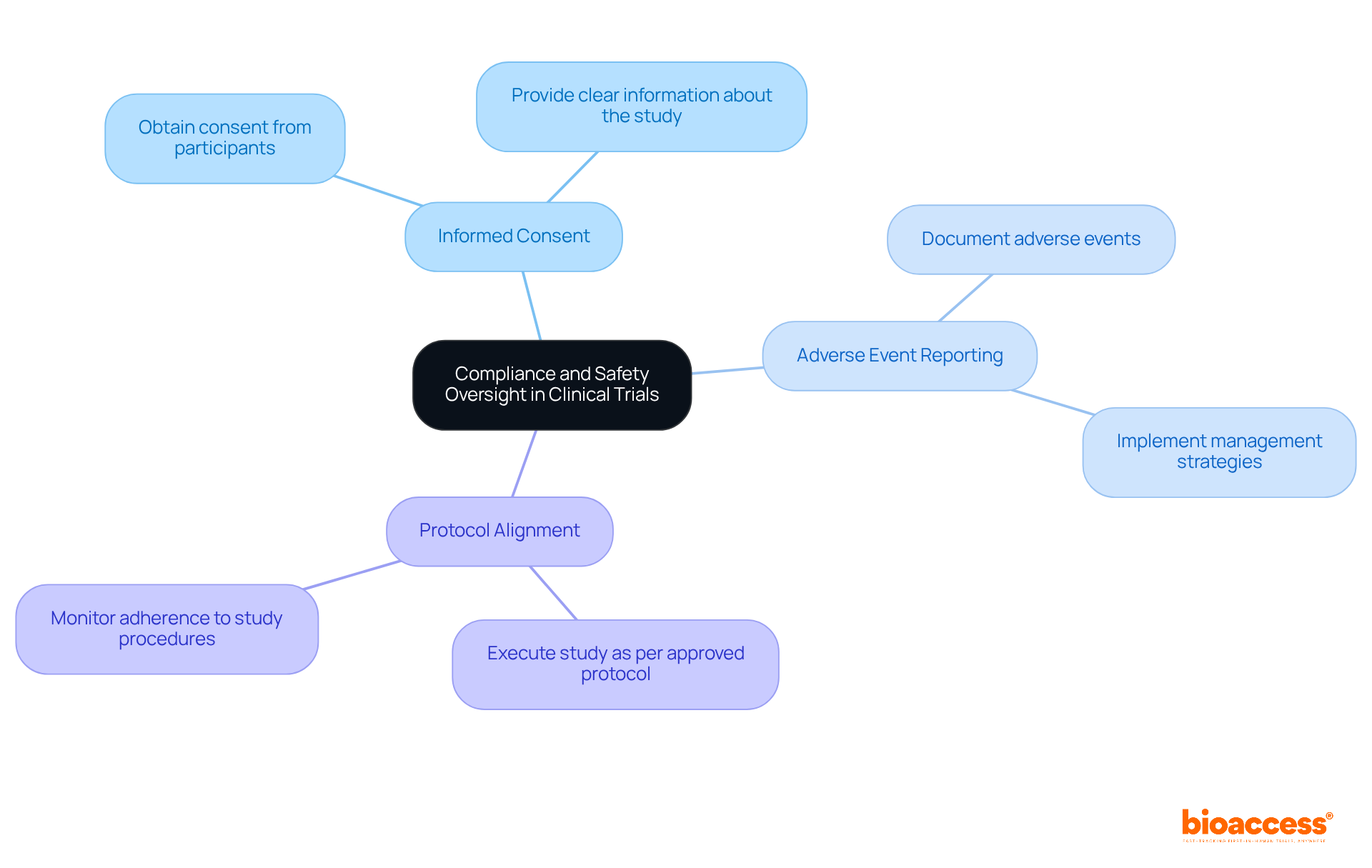
Compliance and safety oversight are essential components of a clinical trial monitor's responsibilities. The role of a clinical trial monitor is pivotal in ensuring that studies adhere to regulatory standards set forth by organizations such as the FDA and EMA. This responsibility includes:
Safety oversight involves continuous assessment of participant health and well-being throughout the study, ensuring that any potential risks are identified and addressed promptly. By upholding rigorous compliance and safety standards, monitors contribute to maintaining the ethical integrity of medical studies and protecting the rights of participants.
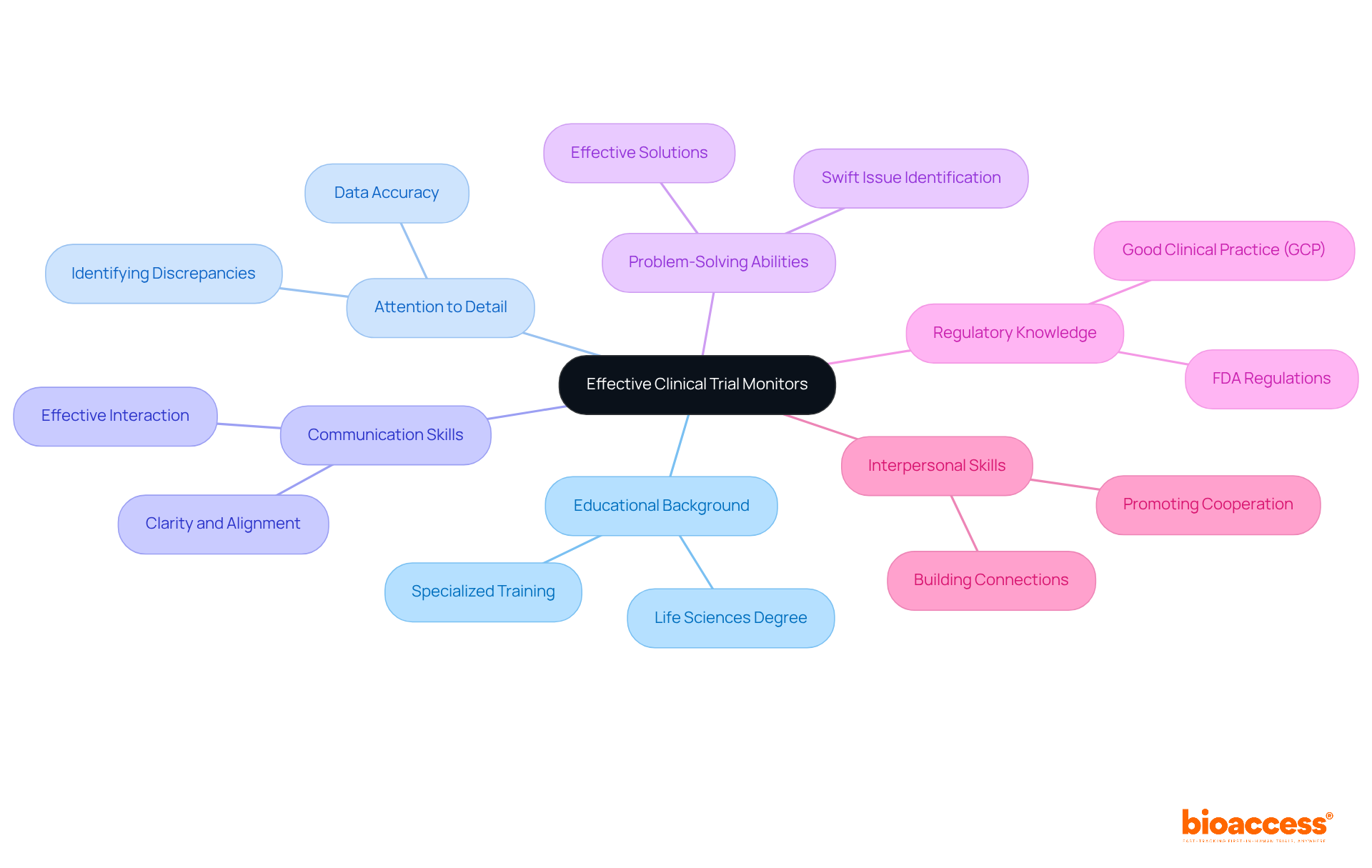
Efficient clinical trial monitors are characterized by a distinctive blend of abilities and credentials that are essential for the success of medical investigations. Key attributes include:
These competencies not only enhance the monitor's effectiveness but also contribute significantly to the overall success of clinical trials, ensuring that they are conducted efficiently and ethically.
The role of a clinical trial monitor is pivotal in the realm of medical research, acting as a crucial safeguard for both data integrity and participant welfare. By ensuring adherence to regulatory standards, ethical guidelines, and research protocols, clinical trial monitors uphold the foundational principles of clinical trials. Their expertise not only facilitates the smooth execution of studies but also fortifies the trust essential for effective research.
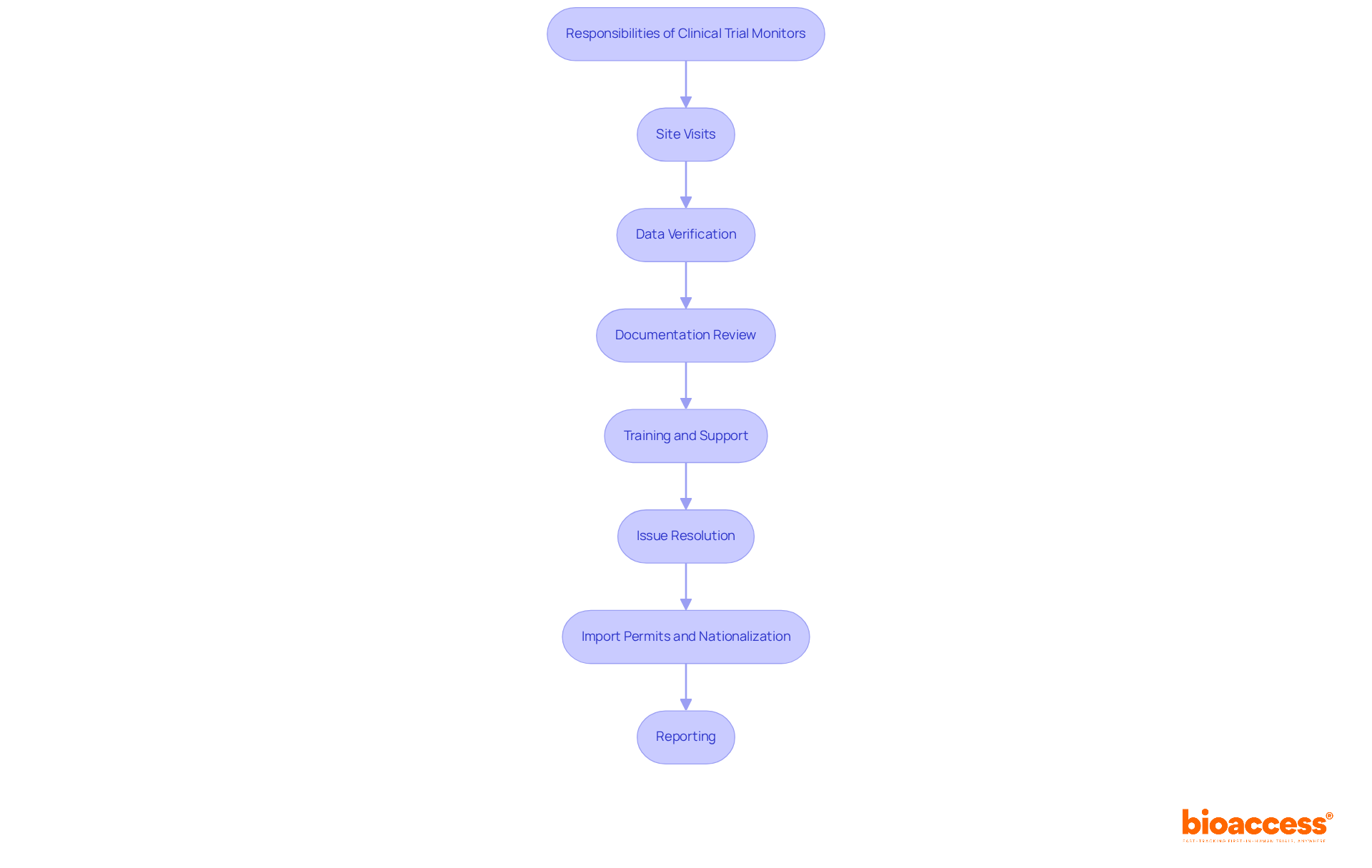
Throughout this article, we have underscored the key responsibilities of clinical trial monitors, which include:
These tasks are indispensable for maintaining the quality and reliability of clinical data. Furthermore, we have highlighted the importance of skills such as attention to detail, communication, and regulatory knowledge, illustrating how these attributes contribute to the overall success of clinical trials.
The significance of clinical trial monitors transcends mere oversight; they are integral to cultivating an environment of ethical research and participant safety. As the demand for innovative medical solutions escalates, the role of clinical trial monitors will continue to evolve. Recognizing their importance in clinical research not only enhances the credibility of studies but also fosters a commitment to ethical practices. It is imperative for stakeholders in the research community to acknowledge and support the vital contributions of clinical trial monitors in advancing medical science.
What is the role of a clinical trial monitor?
A clinical trial monitor, also known as a Clinical Research Associate (CRA), oversees clinical studies to ensure compliance with regulatory requirements, research protocols, and Good Clinical Practice (GCP) guidelines.
Why is the role of a clinical trial monitor important?
The role is crucial for protecting the rights and well-being of participants, ensuring data integrity, and facilitating communication between the sponsor and research site. They also help identify discrepancies in clinical data, which can indicate potential fraud or errors.
How do clinical trial monitors contribute to data integrity?
Clinical trial monitors identify discrepancies in clinical data, which can signal potential fraud or errors. Their effective monitoring practices can lead to early detection of data inconsistencies, preserving the integrity of the experiment.
What ethical responsibilities do clinical trial monitors have?
They ensure that informed consent is obtained from participants and that ethical standards are upheld throughout the study, especially in studies involving vulnerable populations where risks must be justified by potential benefits.
How do recent regulatory updates affect the role of clinical trial monitors?
Recent updates in regulatory frameworks emphasize stringent ethical oversight, reinforcing the necessity of clinical trial monitors in maintaining high standards of participant safety and ethical conduct in medical investigations.
What outcomes can be attributed to the work of clinical trial monitors?
Their contributions facilitate the smooth implementation of studies, uphold participant trust and safety, and ultimately lead to more reliable and impactful outcomes in medical research.