


The role of a Clinical Trial Project Manager is crucial for the successful execution of clinical trials. They oversee planning, coordination, and compliance among diverse stakeholders to ensure efficient study processes. Effective management not only mitigates challenges such as recruitment delays and regulatory changes but also significantly enhances patient participation and retention. Ultimately, this drives advancements in medical research and innovation.
The intricate world of clinical trials is fundamentally anchored by the expertise of clinical trial project managers, who act as the linchpins in securing successful research outcomes. These professionals adeptly navigate a landscape replete with regulatory challenges, budget constraints, and the imperative for effective communication among diverse stakeholders. As the demand for innovative medical solutions intensifies, grasping the multifaceted role of a clinical trial project manager becomes paramount.
What strategies can these managers implement to surmount recruitment hurdles and enhance the efficiency of clinical trials, ultimately propelling advancements in healthcare?
A clinical trial project manager is pivotal in the successful execution of research studies, overseeing their planning, execution, and conclusion. This multifaceted role requires the coordination of diverse teams, budget management, and strict compliance with legal standards. Acting as a vital link among stakeholders—such as sponsors, regulatory bodies, and research sites—the clinical trial project manager ensures that trials are conducted efficiently and effectively.
In 2025, the clinical trial project manager's responsibilities have expanded to include advanced planning, risk management, and stakeholder communication, all of which are essential for navigating the complexities of medical research. With approximately 80% of studies experiencing delays due to recruitment challenges, the CSPM's capacity to implement effective strategies is crucial. Organizations that adopt structured approaches achieve an impressive 92% success rate in meeting their objectives, underscoring the importance of skilled management.
The expertise of a clinical trial project manager is fundamental in enhancing patient participation and retention, which are vital for the success of research studies. Their leadership not only streamlines processes but also significantly elevates the quality of medical research. Effective management can yield cost savings of $25,000 per patient, illustrating the financial benefits of proficient oversight.
Successful examples of medical study management often involve expedited patient recruitment services, facilitating quicker enrollment of treatment-naive groups and resulting in notable efficiency gains. As the demand for medical studies continues to grow, the role of the clinical trial project manager remains crucial in advancing medical knowledge and improving patient outcomes.
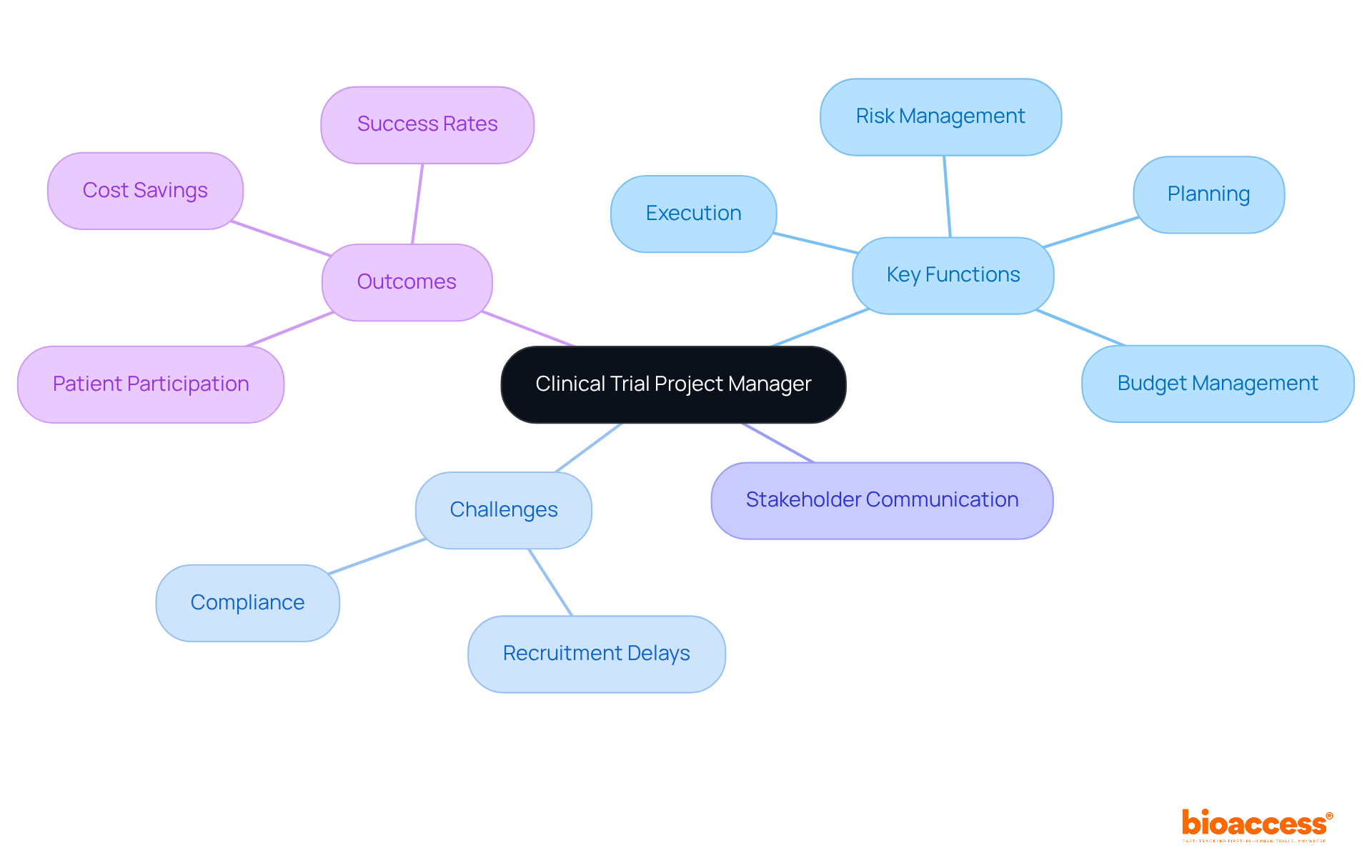
The responsibilities of a Clinical Trial Project Manager encompass several critical areas essential for the successful execution of clinical trials:
Project Planning: Developing comprehensive project plans that detail timelines, resources, and budgets is fundamental. This involves the clinical trial project manager using project management software and templates to outline key milestones and monitor deliverables from protocol development to study closeout.
Leading cross-functional teams: As a clinical trial project manager, it is vital to lead cross-functional teams, including healthcare operations, data management, and regulatory affairs, to ensure seamless collaboration. Regular team meetings and progress reports help maintain alignment on objectives and timelines, fostering a cooperative environment.
Financial oversight: The clinical trial project manager is responsible for overseeing the financial aspects of the study, which includes creating budgets, monitoring expenditures, and ensuring cost-effectiveness. With the average budget for clinical studies in 2025 anticipated to be significant, effective financial supervision is essential for ensuring project viability.
Regulatory compliance: Ensuring adherence to ethical guidelines and regulatory requirements is paramount for maintaining the integrity of the research, a responsibility often overseen by the clinical trial project manager. This includes following a Regulatory Compliance Checklist to meet ICH-GCP, FDA, and EMA standards, which helps mitigate compliance risks.
Risk management: For a clinical trial project manager, recognizing potential risks and applying strategies to reduce them is crucial for maintaining the study's progress. Proactive risk management promotes a culture of vigilance, significantly enhancing the likelihood of success.
Communication facilitation: Facilitating clear communication among stakeholders—including sponsors, investigators, and oversight bodies—is crucial for the clinical trial project manager to keep everyone informed and aligned. Creating a thorough communication strategy from the beginning can avert misunderstandings and delays, improving overall efficiency.
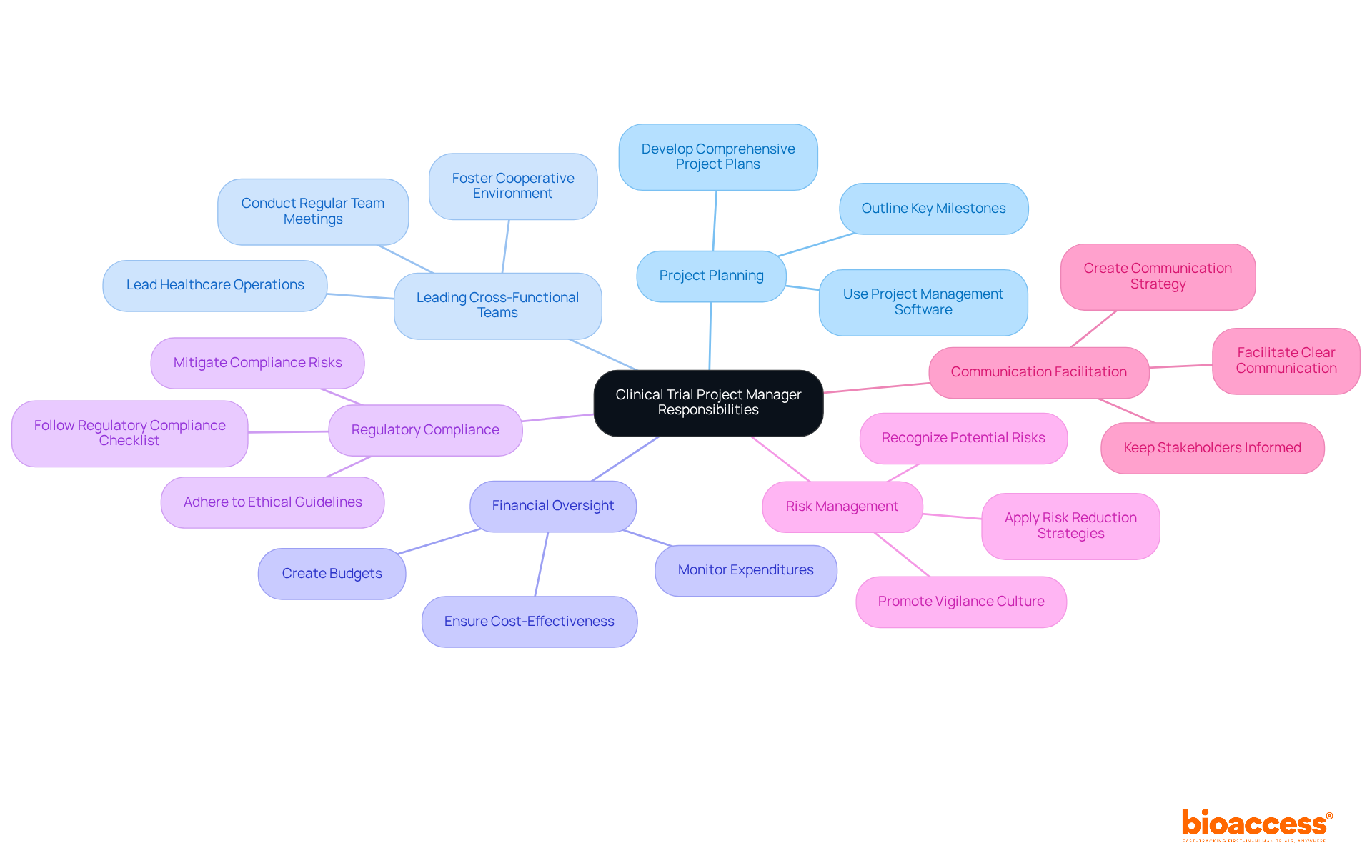
Clinical trial project managers (CTPMs) play a crucial role in the advancement of medical research and innovation. By ensuring that clinical trials are executed efficiently and comply with regulatory standards, the clinical trial project manager facilitates the development of new therapies and medical devices. Their expertise in managing complex tasks leads to timely data collection and analysis, which are crucial for evaluating the safety and efficacy of emerging treatments. Notably, 80% of studies experience delays due to recruitment challenges, highlighting the pivotal role of the clinical trial project manager in addressing these issues and maintaining project timelines.
Furthermore, the role of a clinical trial project manager is to promote collaboration among diverse stakeholders, including researchers, regulatory bodies, and healthcare providers. This integrated approach enhances communication and coordination, ultimately improving patient outcomes and accelerating the introduction of innovative medical solutions. For example, a recent case study demonstrated that the involvement of skilled clinical trial project managers in an Early Feasibility Study significantly streamlined processes and mitigated risks, ensuring that innovative therapies reached patients promptly.
The impact of research study efficiency on medical innovation is profound. As the global medical investigation market is projected to surpass $80 billion by 2025, the demand for proficient clinical trial project managers is on the rise, particularly in fields such as gene therapy and personalized medicine. Their ability to navigate the complexities of medical studies not only drives progress in healthcare technology but also enhances the reliability and validity of research outcomes, ultimately supporting the advancement of healthcare.
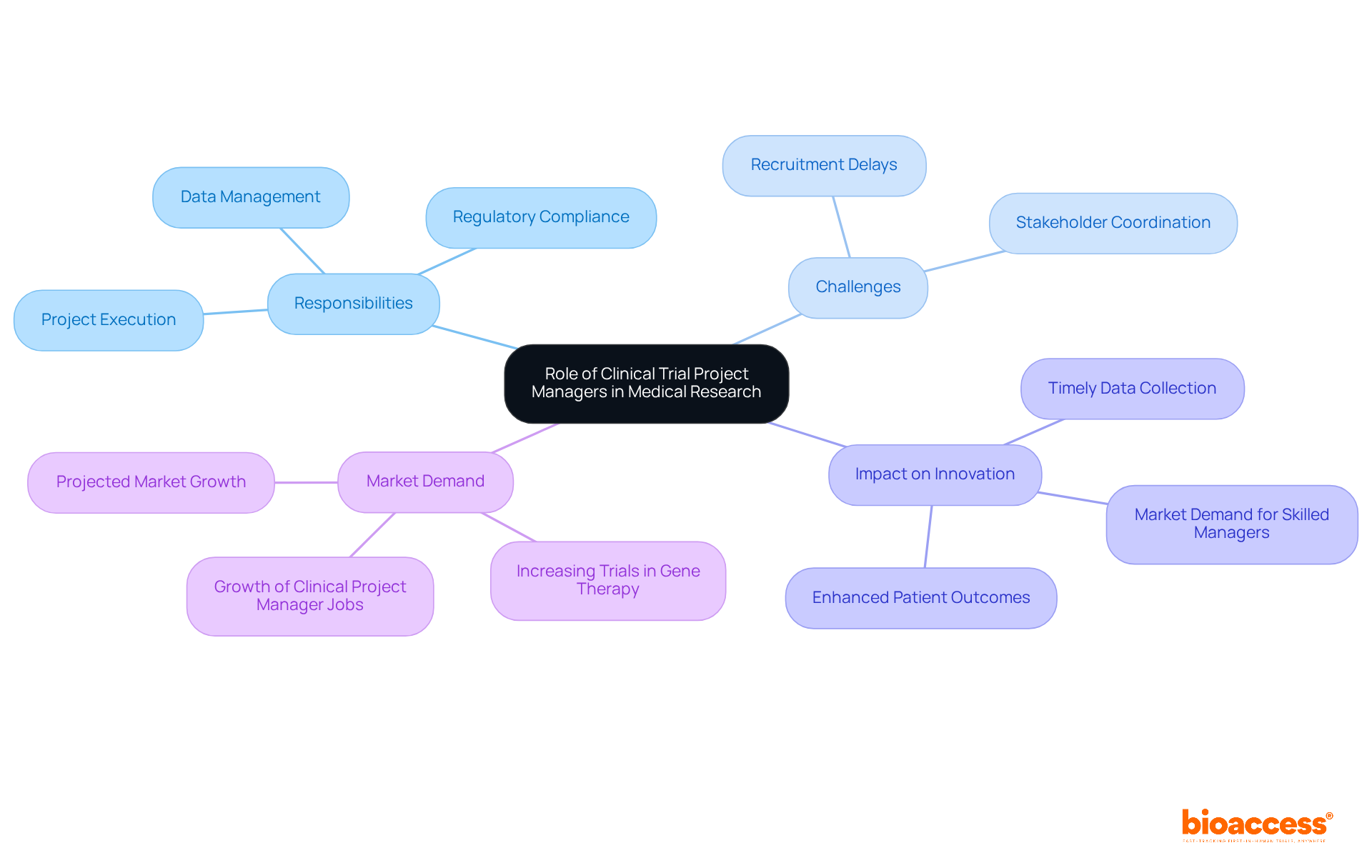
Clinical Trial Project Managers face a multitude of challenges that can significantly impact the success of clinical trials:
Regulatory Changes: The landscape of clinical trial regulations is in a constant state of flux, demanding ongoing vigilance and adaptability. The increasing complexity of protocols, characterized by a rise in the number of endpoints, complicates planning and execution. This dynamic environment requires managers to stay informed and responsive to compliance changes. Bioaccess provides expertise in regulatory affairs, equipping project managers to navigate these changes effectively.
Resource Allocation: Efficient management of limited resources is crucial to ensuring that all components of the experiment receive adequate support. In 2025, the challenge of resource allocation becomes particularly pronounced, as many studies experience enrollment fatigue that can hinder progress. For instance, a Phase III study for Metastatic Colorectal Cancer aimed to enroll 1,250 patients but faced recruitment difficulties due to resource constraints. Bioaccess's feasibility studies and site selection services can optimize resource allocation to address these challenges.
Stakeholder Management: Balancing the diverse interests and expectations of stakeholders—including sponsors, regulatory bodies, and clinical sites—can lead to conflicts and delays. Successfully navigating these relationships is essential for maintaining progress and achieving objectives. The expertise of professionals such as Juan Cuya, MD, is invaluable in managing these relationships efficiently, ensuring alignment between stakeholder interests and study objectives.
Data Management: Accurate and timely data collection is critical, particularly in light of stringent data protection regulations. Ensuring compliance while managing extensive data requires robust systems and processes to mitigate risks associated with data breaches and inaccuracies. Bioaccess's project management services encompass comprehensive reporting on study status and adverse events, aiding in the maintenance of data integrity and compliance.
Patient Recruitment: Attracting and retaining participants presents a significant challenge, especially in competitive therapeutic areas. Innovative recruitment strategies, such as community outreach and tailored engagement efforts, have proven effective in overcoming these obstacles. For example, a recent study on atopic dermatitis successfully coordinated recruitment efforts around children's school schedules, illustrating the importance of flexibility in patient engagement. Bioaccess's support in setup and monitoring can further enhance recruitment initiatives.
By recognizing and addressing these challenges, strategies can be implemented by clinical trial project managers that boost trial efficiency and mitigate risks. Bioaccess's comprehensive services collectively contribute to the successful advancement of medical innovations.
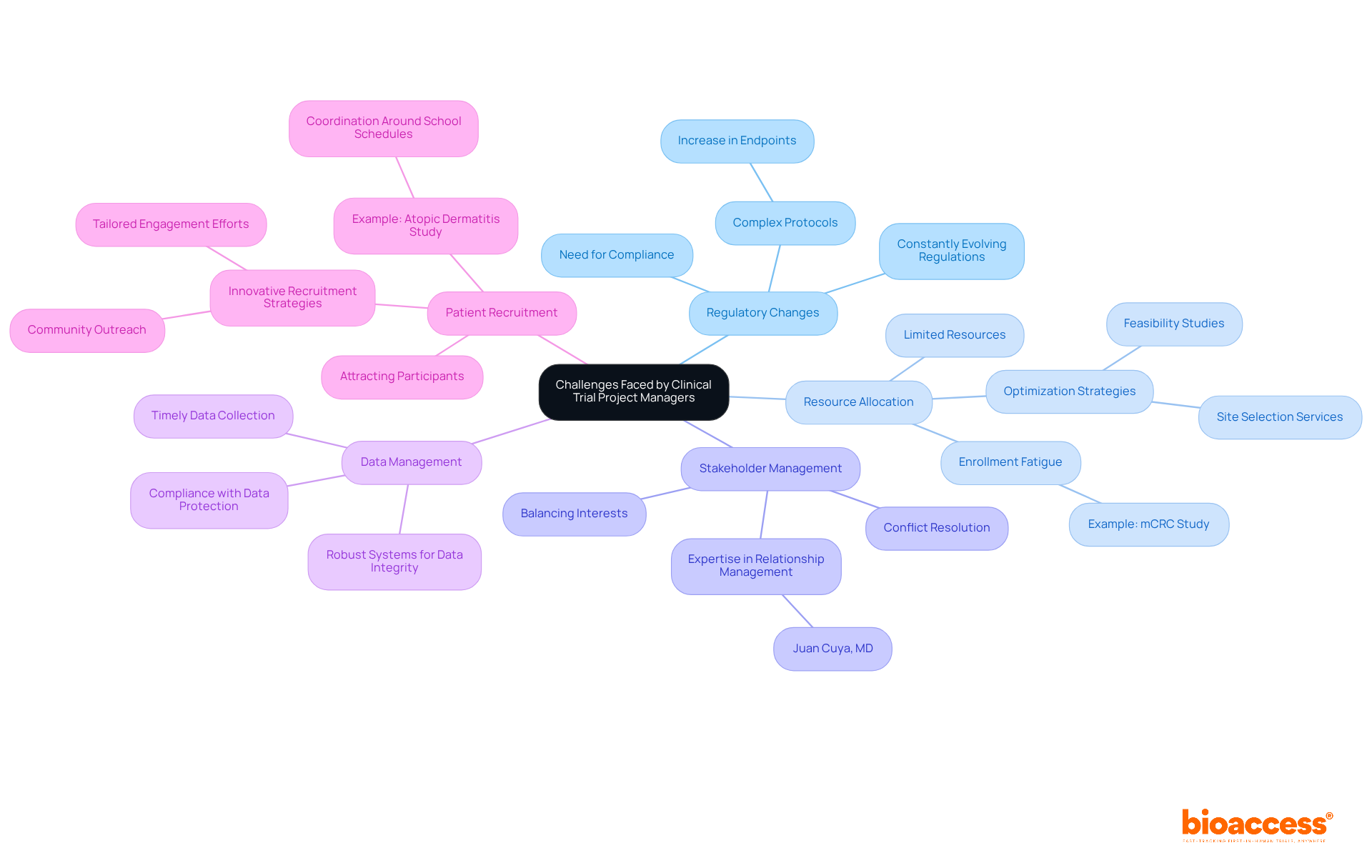
The role of a clinical trial project manager is indispensable in the realm of medical research, serving as a linchpin that connects various stakeholders and ensures that clinical trials are executed with precision and efficiency. This position encompasses not only the management of trial logistics but also demands a keen understanding of regulatory compliance, risk management, and effective communication. As the demand for innovative medical solutions grows, the significance of skilled clinical trial project managers continues to rise.
Throughout the article, key responsibilities of clinical trial project managers have been highlighted, including:
Their expertise in navigating the complexities of clinical trials directly impacts patient recruitment and retention, ultimately enhancing the quality and success rate of research studies. The challenges faced by these professionals—ranging from regulatory changes to resource allocation—underscore the critical nature of their work in overcoming obstacles that can delay or derail studies.
In conclusion, the role of a clinical trial project manager transcends mere oversight; it is about driving innovation and improving patient outcomes within the healthcare landscape. As the global medical investigation market expands, the need for adept project managers will only intensify. Embracing structured approaches and innovative strategies is essential for significant advancements in medical research, making it crucial for organizations to invest in skilled management to navigate the future of clinical trials effectively.
What is the role of a clinical trial project manager?
A clinical trial project manager oversees the planning, execution, and conclusion of research studies, coordinating diverse teams, managing budgets, and ensuring compliance with legal standards.
What are the key responsibilities of a clinical trial project manager in 2025?
In 2025, their responsibilities include advanced planning, risk management, and stakeholder communication, which are essential for navigating the complexities of medical research.
Why is the role of a clinical trial project manager important for recruitment?
Approximately 80% of studies experience delays due to recruitment challenges, making the project manager's ability to implement effective strategies crucial for timely patient enrollment.
How does structured management impact the success of clinical trials?
Organizations that adopt structured approaches achieve a 92% success rate in meeting their objectives, highlighting the importance of skilled management in clinical trials.
What financial benefits can effective clinical trial management provide?
Effective management can yield cost savings of $25,000 per patient, demonstrating the financial advantages of proficient oversight.
How does a clinical trial project manager enhance patient participation and retention?
Their leadership streamlines processes and significantly elevates the quality of medical research, which is vital for the success of research studies.
What are some successful strategies used in medical study management?
Successful strategies often include expedited patient recruitment services, which facilitate quicker enrollment of treatment-naive groups and result in efficiency gains.
Why is the role of a clinical trial project manager crucial for advancing medical knowledge?
As the demand for medical studies grows, the clinical trial project manager plays a key role in improving patient outcomes and advancing medical knowledge through effective trial management.