


The role of a clinical trial sponsor in research is pivotal; they initiate, oversee, and fund clinical studies, ensuring compliance with regulatory and ethical standards while maintaining data integrity and participant safety. Sponsors, primarily from pharmaceutical companies, are crucial in:
These responsibilities collectively influence the success of clinical trials and the advancement of medical knowledge. Their involvement not only ensures that trials are conducted ethically but also supports the generation of reliable data, which is essential for medical progress.
The landscape of clinical research is intricately woven with the pivotal role of clinical trial sponsors, whose influence extends far beyond mere funding. These sponsors—ranging from pharmaceutical giants to academic institutions—are the architects of research initiatives, ensuring that studies are not only scientifically sound but also ethically conducted. However, as the complexities of clinical trials increase, so do the challenges they face in maintaining compliance and safeguarding participant safety.
How do these sponsors navigate the evolving regulatory environment while fostering innovation and trust within the research community?
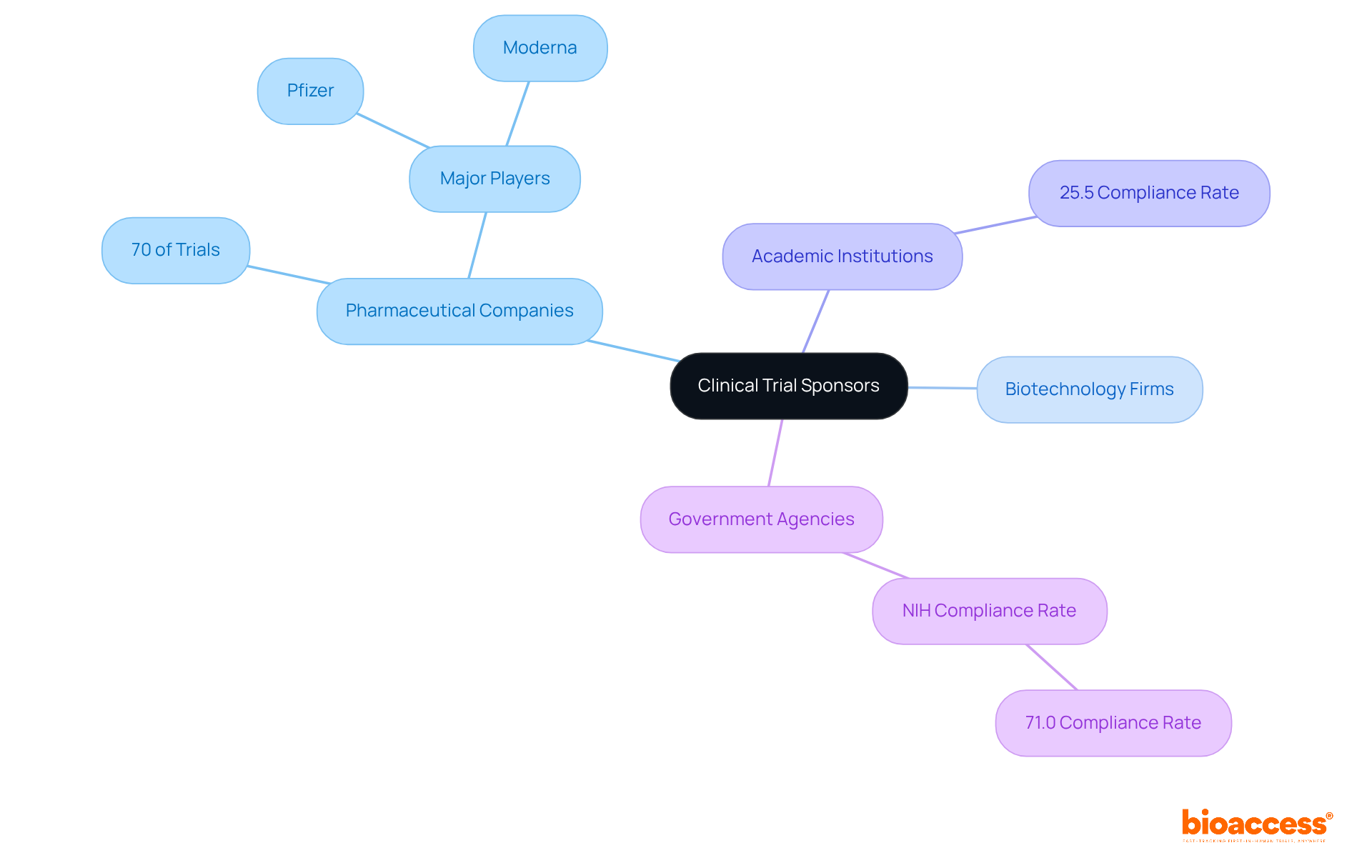
A clinical trial sponsor is a person, institution, or organization responsible for initiating, overseeing, and funding a research project. This encompasses pharmaceutical companies, biotechnology firms, academic institutions, and government agencies. Clinical trial sponsors play a vital role in the design and implementation of medical studies, ensuring compliance with regulatory requirements and ethical standards. They oversee the integrity of the process, which includes data management and the reporting of outcomes.
Notably, pharmaceutical firms account for approximately 70% of all research trials, significantly outpacing other entities such as academic institutions and government bodies. Prominent backers in the Medtech and Biopharma sectors include major pharmaceutical companies like Pfizer and innovative biotech firms like Moderna.
Experts emphasize that the commitment of clinical trial sponsors to scientific rigor, patient safety, and ethical conduct is crucial for research success. As a clinical trial sponsor, their responsibilities extend beyond the initiation of the process; they must also ensure timely reporting of outcomes and maintain transparency throughout, which is essential for fostering trust among stakeholders and participants.
In Latin America, bioaccess® stands out as a leading contract research organization (CRO) specializing in medical device studies. With a focus on Medtech startups, bioaccess® offers tailored solutions, including compliance approval and participant recruitment, ensuring high-quality and cost-effective services that facilitate expedited study results.
Clinical research funders serve as a cornerstone in the research landscape, fundamentally shaping the foundation of clinical studies. They engage in collaboration with researchers, governing bodies, and ethics committees to ensure that studies are meticulously designed, balancing both effectiveness and ethical considerations. Beyond providing essential funding—vital in the highly regulated domains of Medtech, Biopharma, and Radiopharma—these backers play an integral role in strategic planning and protocol development. Their vigilant monitoring of trial progress ensures adherence to legal standards and compliance throughout the study's lifecycle.
The funding strategies employed by backers can vary significantly, including the pursuit of grants, attracting private investments, and fostering collaborations with healthcare organizations. This financial backing transcends a mere transactional relationship; it nurtures a collaborative environment where funders and researchers align research objectives with regulatory requirements. Such collaboration is crucial, as it enhances the likelihood of success by ensuring studies are not only well-designed but also adequately funded.
Expert insights underscore the profound impact that research funders have on study success rates. By actively engaging in the design and execution of studies, these backers mitigate risks and bolster data integrity. Their continuous monitoring and oversight are indispensable for safeguarding participant safety and ensuring the reliability and actionability of the data collected. This comprehensive involvement not only propels the advancement of medical knowledge but also aids in the development of innovative therapies that can significantly enhance patient care.

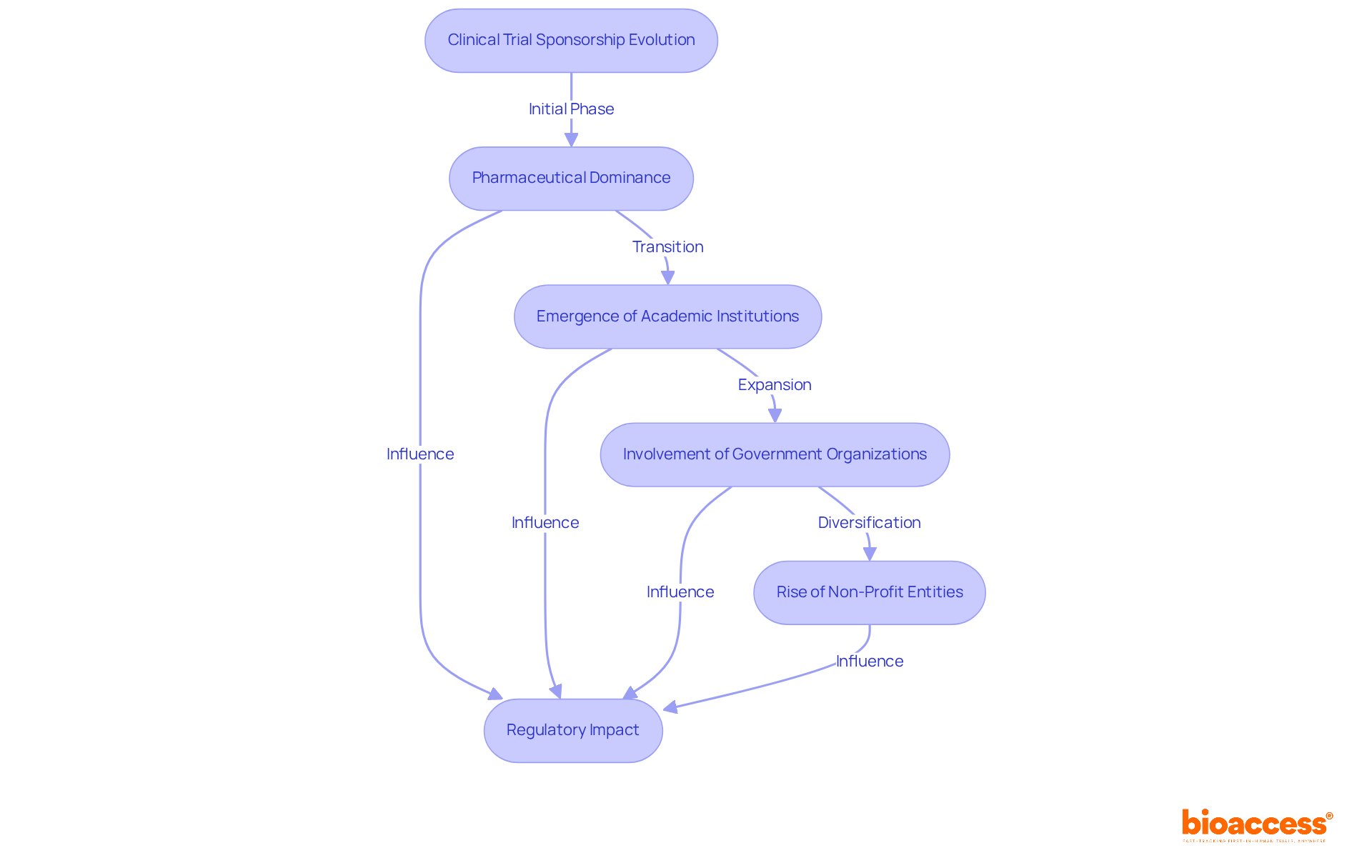
The landscape of research trial sponsorship has undergone substantial transformation in recent decades, particularly for clinical trial sponsors. Initially dominated by pharmaceutical companies, the role of sponsors has expanded to include academic institutions, government organizations, and non-profit entities. This diversification reflects the increasing complexity of clinical research and the necessity for a range of expertise.
Furthermore, the implementation of stringent oversight frameworks, such as the FDA's Good Clinical Practice guidelines, has significantly shaped the responsibilities of the clinical trial sponsor. These regulations mandate that backers ensure trials are scientifically valid and ethically sound, navigating a complex environment of compliance and oversight.
Over the past ten years, changes in governance frameworks have further influenced sponsorship dynamics, underscoring the importance of transparency and accountability in clinical research. As a result, sponsors today must adeptly balance scientific rigor with ethical considerations, adapting to a regulatory landscape that prioritizes patient safety and data integrity.
Responsibilities held by clinical trial sponsors are essential and pivotal to the success of research initiatives.
Study Design and Protocol Development: Sponsors are responsible for formulating the study and developing the protocol, which outlines the study's objectives, methodology, and statistical analysis plan. This essential step is vital for the clinical trial sponsor to guarantee that the trial is scientifically valid and fulfills compliance expectations.
Regulatory Compliance: A fundamental duty of those providing support is to ensure adherence to all applicable regulations and ethical guidelines. This includes navigating complex compliance landscapes, which can present challenges such as varying international regulations and the need for timely approvals. Bioaccess® can aid backers in this area by offering prompt and dependable regulatory approval, contributing to the efficiency of the process and minimizing delays. For example, the typical funding provided by backers for research studies frequently indicates the considerable resources needed to address these compliance requirements. Furthermore, roughly 80% of medical studies are postponed or terminated due to recruitment issues, highlighting the difficulties that clinical trial sponsors face in ensuring timely advancement.
Funding and Resource Allocation: Securing sufficient financing is essential, as clinical studies can cost between USD 266 million and USD 802 million to introduce a new drug to market. Clinical trial sponsors must effectively distribute these resources to ensure the project's success, often involving strategic partnerships and collaborations to enhance funding opportunities. This careful allocation is essential to meet the financial demands of compliance and operational needs.
Monitoring and Reporting: Sponsors are responsible for closely observing the study's progress, ensuring data integrity, and reporting results to regulatory authorities and stakeholders. This oversight is critical, as delays in reporting can lead to significant financial repercussions, costing sponsors between $600,000 and $8 million for each day of delay.
Safety Oversight: Ensuring participant safety is paramount. Sponsors must monitor adverse events and implement necessary safety measures throughout the study. This involves keeping open channels of communication with participants, as research indicates that 90.4% of chronic disease patients appreciate conversations with research coordinators and nurses. Bioaccess® enhances these interactions by linking innovative startups with leading research locations, facilitating effective communication and boosting patient recruitment.
These responsibilities underscore the vital role of the clinical trial sponsor in the successful execution of clinical trials, ultimately influencing patient outcomes and the advancement of medical knowledge.

Clinical trial sponsors are the backbone of medical research, playing an indispensable role in the initiation, oversight, and funding of clinical studies. Their commitment to ensuring scientific integrity, patient safety, and ethical standards is vital for the successful execution of research initiatives. By navigating complex regulatory landscapes and fostering collaboration among stakeholders, sponsors not only enhance the credibility of clinical trials but also contribute significantly to the advancement of medical knowledge.
Key insights throughout the article highlight the multifaceted responsibilities of clinical trial sponsors, including:
These elements underscore how sponsors' active engagement shapes the trajectory of clinical research, ensuring that studies are not only well-funded but also meticulously designed to meet ethical and scientific standards. The evolution of sponsorship dynamics, influenced by regulatory changes and the growing diversity of funding sources, reflects the increasing complexity of the research landscape.
The significance of clinical trial sponsors extends beyond the confines of individual studies; their influence resonates throughout the healthcare ecosystem. As the demand for innovative therapies continues to rise, the importance of fostering robust partnerships between sponsors, researchers, and regulatory bodies cannot be overstated. Embracing this collaborative spirit will not only enhance research outcomes but also ensure that medical advancements are both safe and effective for patients. Engaging with clinical trial sponsors can catalyze progress in medical research, ultimately leading to improved patient care and outcomes.
What is a clinical trial sponsor?
A clinical trial sponsor is a person, institution, or organization responsible for initiating, overseeing, and funding a research project, which includes pharmaceutical companies, biotechnology firms, academic institutions, and government agencies.
What role do clinical trial sponsors play in medical studies?
Clinical trial sponsors play a vital role in the design and implementation of medical studies, ensuring compliance with regulatory requirements and ethical standards, overseeing the integrity of the process, data management, and reporting of outcomes.
What percentage of research trials are sponsored by pharmaceutical firms?
Pharmaceutical firms account for approximately 70% of all research trials, significantly outpacing other entities such as academic institutions and government bodies.
Who are some prominent sponsors in the Medtech and Biopharma sectors?
Major pharmaceutical companies like Pfizer and innovative biotech firms like Moderna are prominent sponsors in the Medtech and Biopharma sectors.
What responsibilities do clinical trial sponsors have beyond initiating the process?
Beyond initiation, clinical trial sponsors are responsible for ensuring timely reporting of outcomes and maintaining transparency throughout the research process, which is essential for fostering trust among stakeholders and participants.
What is bioaccess® and what services do they provide?
bioaccess® is a leading contract research organization (CRO) in Latin America that specializes in medical device studies, offering tailored solutions such as compliance approval and participant recruitment, ensuring high-quality and cost-effective services for Medtech startups.