


Understanding the complexities of clinical research can be daunting, especially when navigating the regulatory pathways involved in CINMED submissions. Contract Research Organizations (CROs) are essential partners in this process, providing the expertise necessary to streamline research and enhance the likelihood of successful outcomes. With numerous CROs available, sponsors must carefully consider how to select the right one to meet their unique needs and drive their projects toward success.
In the ever-evolving Medtech landscape, the role of CROs becomes increasingly significant. They not only facilitate compliance with regulations but also help sponsors overcome key challenges in clinical research. By leveraging their knowledge and experience, CROs can significantly improve the efficiency and effectiveness of research initiatives.
Ultimately, collaboration with the right CRO can make all the difference in achieving successful clinical outcomes. Sponsors should take the time to evaluate their options, ensuring they choose a partner that aligns with their goals and can navigate the complexities of the clinical research process.
Contract Research Organizations (CROs) have a pivotal role of CROs in cinmed submissions by supporting the pharmaceutical, biotechnology, and medical device industries during the research phase. Their comprehensive services encompass several key functions that are essential for successful clinical trials.
Study Design: CROs are instrumental in crafting clinical trial designs that not only adhere to regulatory standards but also ensure scientific validity. This foundation is crucial for achieving successful outcomes in research.
Regulatory Compliance: Navigating the intricate regulatory landscape is no small feat. The role of CROs in cinmed submissions involves preparing the necessary documentation and submissions to regulatory agencies, which facilitates the approval process. For instance, Bioaccess accelerates regulatory approval timelines, achieving compliance in as little as 6-8 weeks-significantly faster than the typical 6-12 months seen in the US and EU.
Site Management: Effective site management is another critical function of CROs. They oversee research locations, managing site selection, initiation, and monitoring to ensure strict adherence to protocols and schedules. Bioaccess excels in the feasibility and selection of research sites and principal investigators, streamlining the study setup and approval processes.
Data Management: CROs are responsible for the meticulous collection, management, and analysis of data, ensuring data integrity and compliance with Good Clinical Practice (GCP) standards. This attention to detail is vital for the credibility of research findings.
Patient Recruitment: Utilizing extensive networks, CROs assist in enrolling suitable participants, a key element for the success of research studies. Bioaccess has shown the capability to enroll treatment-naive cardiology or neurology cohorts 50% faster than Western sites, effectively addressing common recruitment challenges faced by medical device startups.
By fulfilling these essential roles, the role of CROs in cinmed submissions allows sponsors to concentrate on their core strengths while ensuring that research studies are conducted effectively and ethically. Bioaccess's comprehensive approach not only addresses regulatory challenges but also enhances project management and reporting, establishing it as a valuable ally for startups navigating the complexities of trial processes.
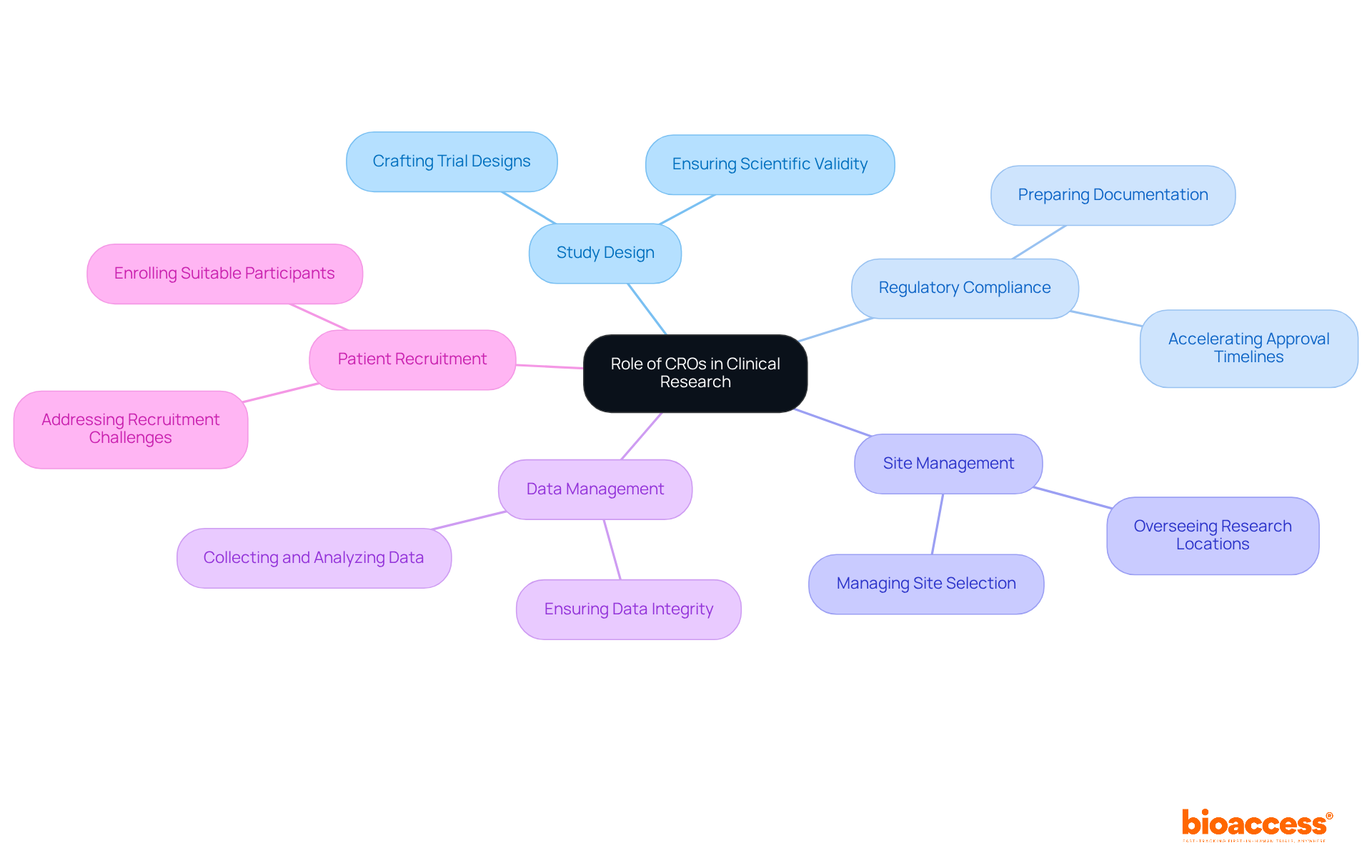
CROs play a crucial role in the CINMED submission process, encompassing several key responsibilities that significantly enhance the likelihood of successful outcomes:
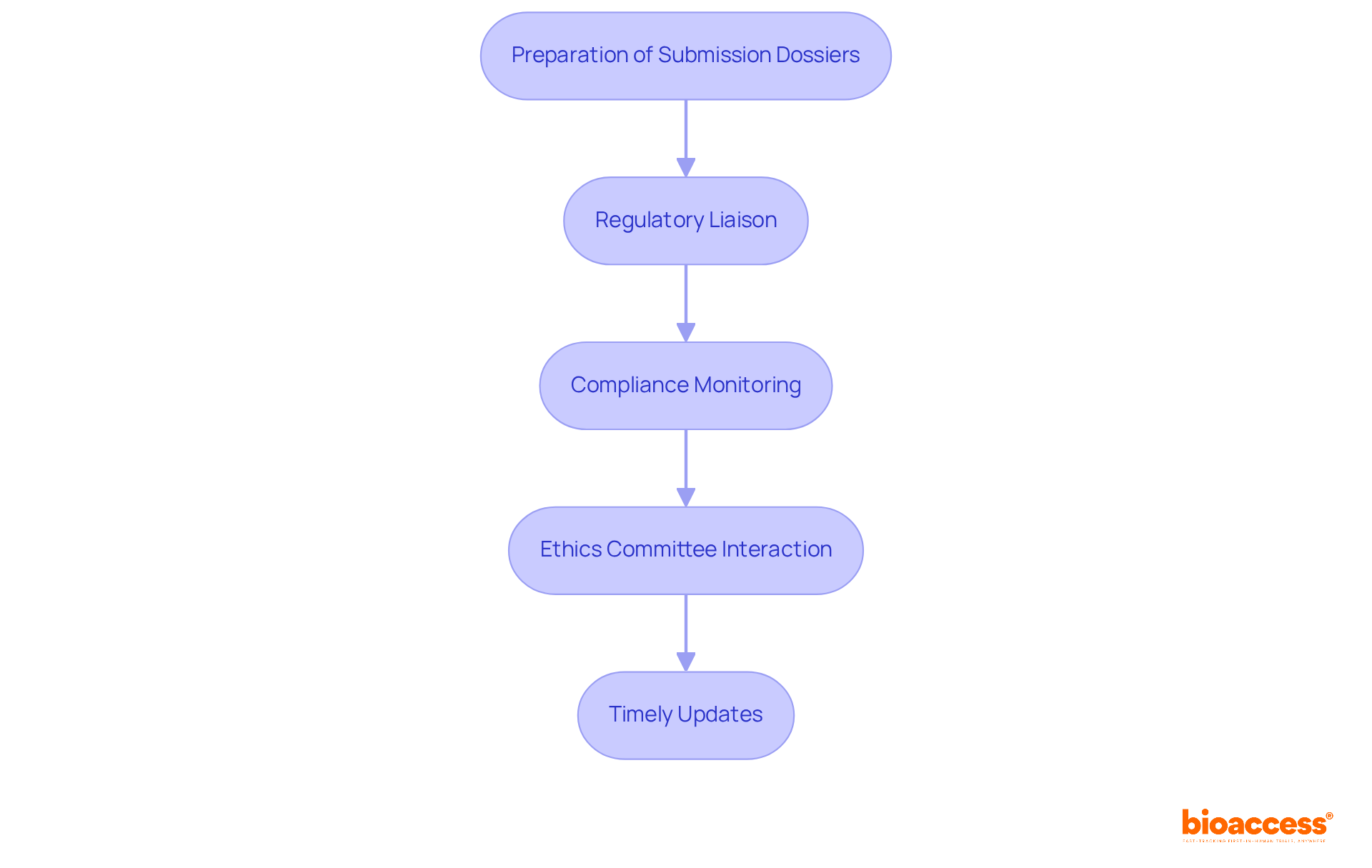
Preparation of Submission Dossiers: CROs meticulously compile comprehensive submission documents, including clinical study reports, investigator brochures, and informed consent forms. This thorough preparation is essential; studies indicate that well-structured dossiers significantly improve approval rates, with 73% of submissions ultimately receiving FDA approval.
Regulatory Liaison: Acting as intermediaries between sponsors and regulatory authorities, clinical research organizations facilitate effective communication and address inquiries that may arise during the evaluation. Their expertise in navigating regulatory landscapes is vital, especially considering that the median delay for applications requiring resubmissions can reach 435 days.
Compliance Monitoring: Contract research organizations ensure adherence to all regulatory requirements throughout the clinical study, conducting audits and inspections to maintain compliance. This proactive approach mitigates risks associated with non-compliance, which can lead to costly delays in the approval process.
Ethics Committee Interaction: By managing submissions to ethics committees, clinical research organizations ensure that all ethical considerations are thoroughly addressed and approved before trials commence. This step is critical in maintaining the integrity of the research and protecting participant rights.
Timely Updates: Clinical research organizations provide regular updates to sponsors regarding the status of submissions, ensuring transparency and prompt responses to regulatory inquiries. This continuous communication builds trust and enables sponsors to make informed choices throughout the submission phase.
The role of CROs in CINMED submissions significantly enhances the efficiency and effectiveness of the submission process through these responsibilities, ultimately contributing to the successful advancement of innovative therapies.
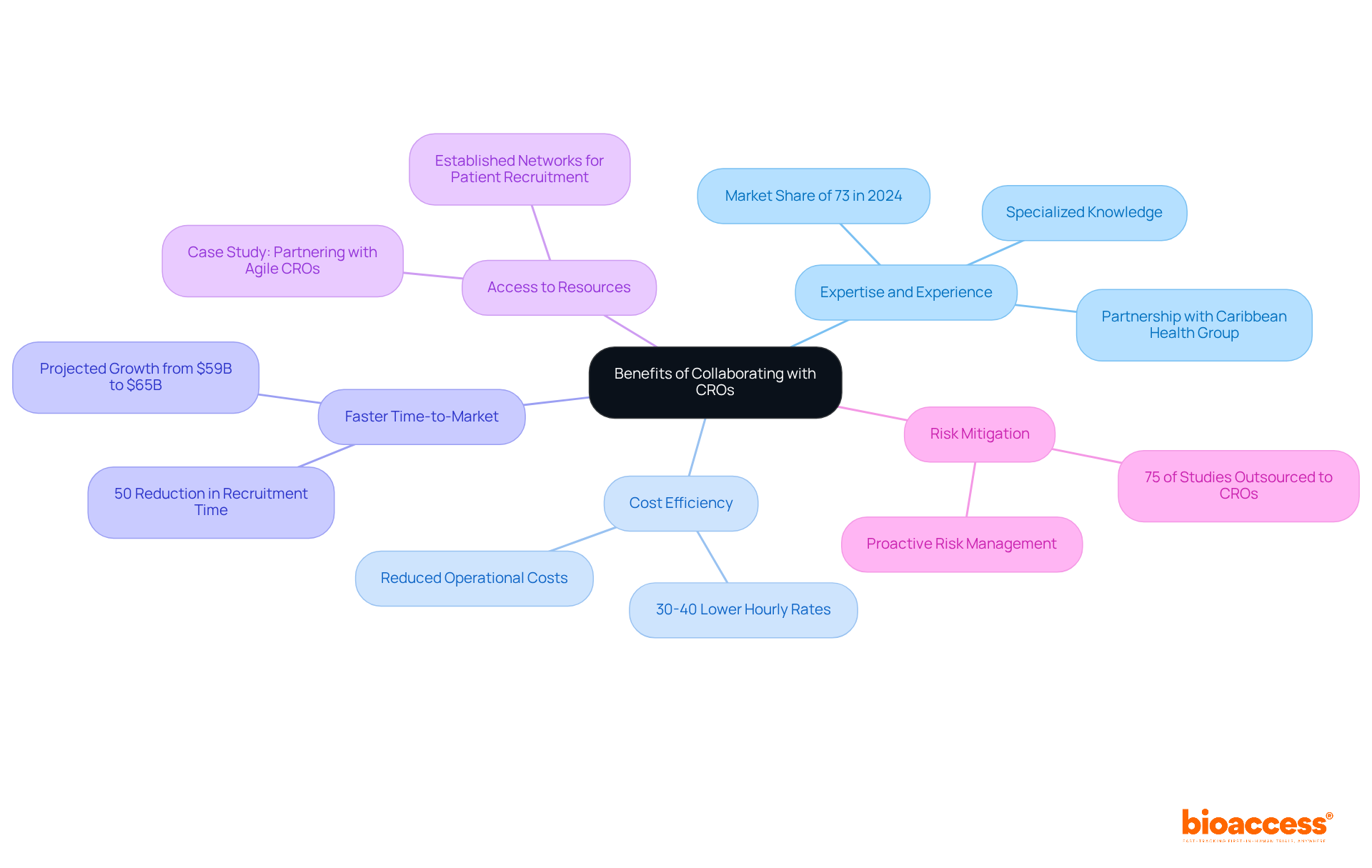
Collaborating with a Contract Research Organization (CRO) offers numerous advantages for sponsors, including:
Expertise and Experience: CROs possess specialized knowledge in clinical trial management, regulatory affairs, and data analysis, significantly enhancing trial quality. Their proficiency is underscored by the medical segment's substantial market share of 73% in 2024, reflecting the importance of expert-led studies. Notably, bioaccess™ has partnered with Caribbean Health Group to leverage this expertise, aiming to position Barranquilla as a leading destination for clinical trials in Latin America, with the support of Colombia's Minister of Health.
Cost Efficiency: Outsourcing to a CRO allows sponsors to reduce operational costs associated with hiring and training in-house staff. Smaller contract research organizations can offer hourly rates for critical roles that are 30-40% lower than those at larger counterparts, making them an appealing option for budget-conscious biotech firms.
Faster Time-to-Market: Contract research organizations streamline processes, enabling quicker patient recruitment and regulatory submissions. This acceleration is vital, as the global CRO market is projected to grow from $59 billion in 2024 to $65 billion in 2025, driven by the increasing demand for efficient drug development solutions. A collaboration with GlobalCare Clinical Trials has resulted in over a 50% reduction in recruitment time and a remarkable 95% retention rate, showcasing the effectiveness of such partnerships.
Access to Resources: Contract research organizations frequently possess established networks and resources that aid in patient recruitment and site selection, enhancing study efficiency. For example, collaborations with nimble contract research organizations have been demonstrated to improve the pace and efficacy of clinical studies, enabling small biotech companies to manage the intricacies of drug development more successfully. A case study titled 'Partnering with Agile Contract Research Organizations for Clinical Trial Success' illustrates these benefits in detail.
Risk Mitigation: With their knowledge, clinical research organizations can identify potential risks early in the study process, enabling proactive management and resolution. This capability is essential, particularly as biopharmaceutical firms increasingly delegate nearly 75% of research studies to contract research organizations to minimize development time and expenses.
These advantages position CROs, such as bioaccess™, as essential allies in the research landscape, particularly highlighting the role of CROs in CINMED submissions, where quality and efficiency are crucial. Testimonials from industry leaders, such as Bill Andrews, Ph.D., President & CEO of Sierra Sciences, further emphasize the value of partnering with bioaccess™ and its dedication to excellence in research studies.
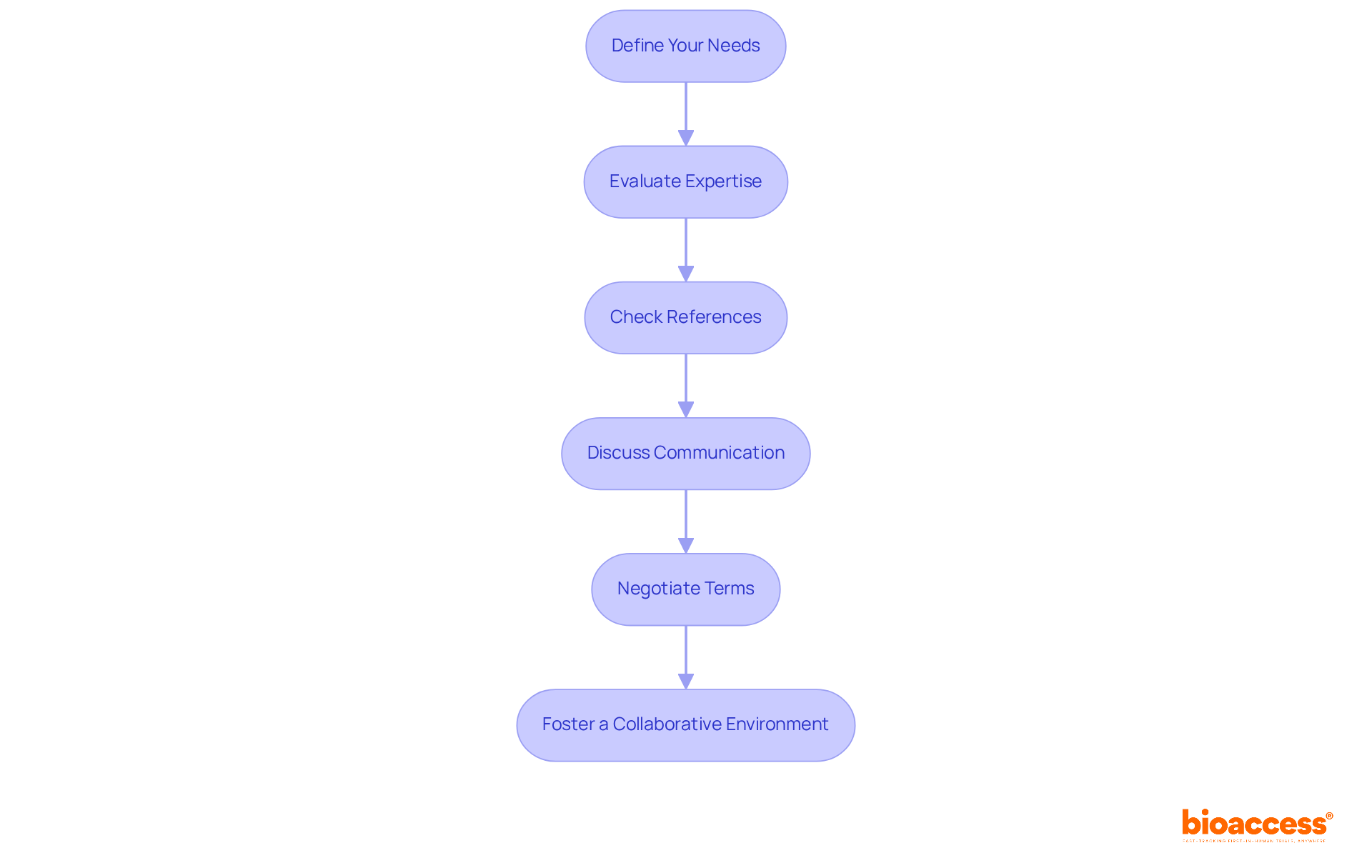
Selecting the right Contract Research Organization (CRO) is crucial for understanding the role of CROs in CinMed submissions for the success of your clinical trial. This process involves several essential steps that can significantly impact your research outcomes.
Define Your Needs: Start by clearly outlining the specific requirements of your clinical trial, including therapeutic area, study design, and regulatory needs. This foundational step ensures that the selected CRO can meet your unique demands.
Evaluate Expertise: Next, assess potential CROs based on their experience in your specific therapeutic area and their track record with similar studies. The role of CROs in CinMed submissions significantly influences the success rates of research studies. Those with specialized knowledge often produce better results. For instance, bioaccess® boasts over 20 years of experience in managing medical device research trials, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF). Additionally, bioaccess® provides accelerated medical device clinical study services in Latin America, offering tailored solutions that navigate the region's unique regulatory landscape.
Check References: It's also vital to request references from previous clients to gauge the CRO's performance and reliability. This practice is crucial, as 81% of responders utilize standardized documents for assessing criteria during the CRO selection procedure, ensuring informed decisions.
Discuss Communication: Establishing clear communication protocols is essential to ensure that both parties are aligned on expectations and timelines. Effective communication fosters a collaborative environment where challenges can be addressed promptly.
Negotiate Terms: Discuss and agree on contract terms, including timelines, deliverables, and budget, to avoid misunderstandings later. A well-defined agreement helps mitigate risks associated with project delays and budget overruns.
Foster a Collaborative Environment: Finally, encourage open communication and teamwork throughout the evaluation process. Organizations that promote collaboration are five times more likely to achieve high performance, underscoring the significance of teamwork in research.
By adhering to these guidelines, sponsors can significantly enhance their chances of selecting a CRO like bioaccess® that aligns with their goals and recognizes the role of CROs in CinMed submissions to foster a successful partnership. This ultimately leads to more efficient and effective clinical trials.
The significance of Contract Research Organizations (CROs) in CINMED submissions is paramount. They are essential partners in the clinical research landscape, offering expertise that helps navigate complex regulatory environments. By managing critical tasks like study design, regulatory compliance, and data management, CROs allow sponsors to concentrate on their core competencies, ensuring that clinical trials are conducted effectively and ethically.
Throughout this discussion, several key functions of CROs have been highlighted. These include:
The advantages of collaborating with CROs extend beyond mere efficiency; they encompass:
These elements are vital for the success of clinical trials. Moreover, the importance of selecting the right CRO and fostering a collaborative relationship cannot be overstated, as these factors significantly influence research outcomes.
In conclusion, partnering with a CRO represents a strategic advantage in the competitive field of clinical research. As the demand for innovative therapies continues to rise, understanding and leveraging the role of CROs in CINMED submissions will be crucial for sponsors aiming to enhance their chances of success. By investing in the right collaborations and adhering to best practices in CRO selection, stakeholders can ensure that their clinical trials not only meet regulatory standards but also contribute to the advancement of medical science.
What is the primary role of Contract Research Organizations (CROs) in clinical research?
CROs play a pivotal role in supporting the pharmaceutical, biotechnology, and medical device industries during the research phase by providing comprehensive services essential for successful clinical trials.
How do CROs contribute to study design?
CROs are instrumental in crafting clinical trial designs that adhere to regulatory standards and ensure scientific validity, which is crucial for achieving successful research outcomes.
What is the role of CROs in regulatory compliance?
CROs help navigate the regulatory landscape by preparing necessary documentation and submissions to regulatory agencies, facilitating the approval process. For example, Bioaccess can achieve compliance in as little as 6-8 weeks, significantly faster than the typical 6-12 months.
What functions do CROs perform in site management?
CROs oversee research locations by managing site selection, initiation, and monitoring to ensure adherence to protocols and schedules, thus streamlining the study setup and approval processes.
How do CROs handle data management?
CROs are responsible for the meticulous collection, management, and analysis of data, ensuring data integrity and compliance with Good Clinical Practice (GCP) standards, which is vital for the credibility of research findings.
What role do CROs play in patient recruitment?
CROs assist in enrolling suitable participants for research studies using their extensive networks, which is key to the success of these studies. For instance, Bioaccess can enroll treatment-naive cardiology or neurology cohorts 50% faster than Western sites.
How do CROs benefit sponsors during clinical trials?
By fulfilling essential roles, CROs allow sponsors to focus on their core strengths while ensuring that research studies are conducted effectively and ethically, thereby enhancing project management and reporting.