


Understanding reliability in scientific research is crucial for ensuring that measurements yield consistent and trustworthy results. As researchers build upon one another's findings, the reliability of their methods stands as a cornerstone of scientific integrity. But what occurs when measurements are inconsistent or unreliable? This article explores the scientific definition of reliability, its critical importance in research, and the various types of reliability that researchers must consider to enhance the credibility and impact of their studies. By delving into these aspects, we aim to underscore the necessity of reliability in fostering trust within the scientific community.
The scientific definition of reliability in scientific research is crucial, as it refers to the consistency and stability of measurements across time and contexts. This concept evaluates how well a measurement tool produces the same outcomes under stable conditions. For example, when a researcher measures a variable multiple times and consistently obtains similar results, that measurement is deemed reliable. This principle is vital as it relates to the scientific definition of reliability, underpinning the validity of study results and ensuring they can be reproduced and trusted.
Dependability can be assessed through various methods, including:
Each of these methods serves to confirm that the evaluation tools are indeed trustworthy. In the Medtech landscape, understanding and applying these reliability measures can significantly impact clinical research outcomes. By ensuring that measurement tools are reliable, researchers can address key challenges and enhance the credibility of their findings.
In summary, the scientific definition of reliability is crucial in scientific research and cannot be overstated. It not only supports the validity of results but also fosters collaboration among researchers, paving the way for more robust and reproducible studies. As we move forward, it is essential to prioritize the development and application of reliable measurement tools in clinical research.

The importance of reliability in studies cannot be overstated. Dependable measurements are the bedrock of scientific knowledge, which aligns with the scientific definition of reliability, ensuring that study findings are consistent and replicable. When researchers present reliable results, they lay a solid foundation for subsequent studies, allowing others to build upon their findings. In clinical studies, for instance, reliable data directly correlates with improved patient outcomes, as it guarantees that treatments are based on consistent and credible evidence.
High dependability, reflecting the scientific definition of reliability, not only bolsters the credibility of studies but also fosters confidence among stakeholders, including regulatory organizations, funding entities, and the public. As Vance W. Berger pointed out, tests grounded in randomization serve as robust alternatives to traditional likelihood-based tests, underscoring the critical role of randomization as a regulatory necessity in enhancing study credibility. Conversely, a lack of dependability, as outlined in the scientific definition of reliability, can undermine the credibility of study results, leading to misinterpretations and ineffective applications in practice.
Recent advancements in methodologies, such as randomization and double-blind designs, have become essential in enhancing study trustworthiness, minimizing selection bias, and ensuring comparability among treatment groups. These trends highlight the vital importance of the scientific definition of reliability in achieving meaningful and impactful healthcare outcomes. For example, a study on mobile health applications for COPD management revealed that reliable data collection improved patient compliance with treatment and overall mood, showcasing the tangible benefits of trustworthy findings.
Moreover, the historical evolution of study dependability, including the emergence of statistical methods like Cronbach's alpha, illustrates the ongoing advancement of criteria that enhance study quality. Together, these elements underscore the indispensable role of trustworthiness in clinical studies.
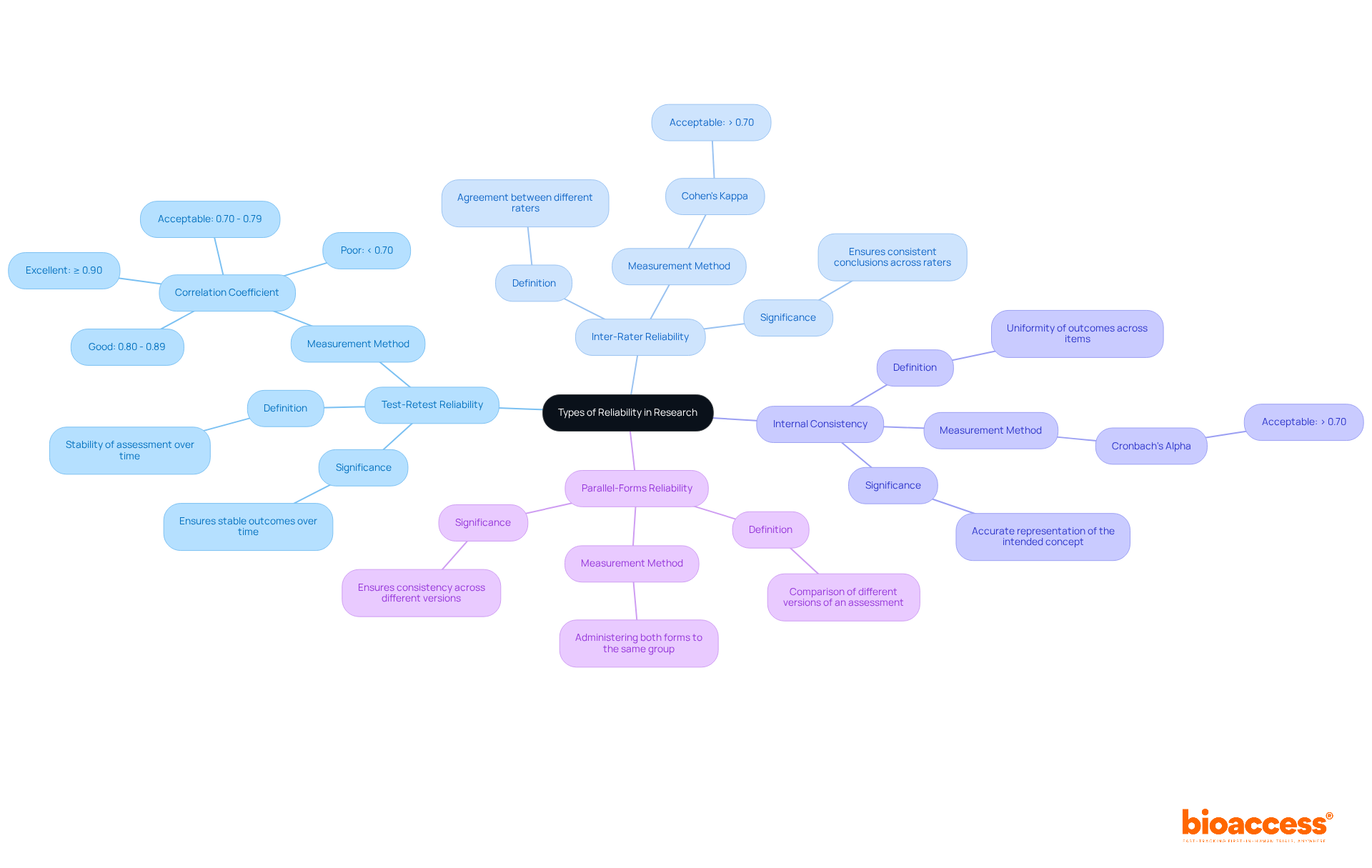
Researchers can assess several types of reliability to ensure the consistency of their measurements, each serving a distinct purpose in validating research findings:
Test-Retest Reliability: This type assesses the stability of an assessment over time. By conducting the same assessment on the same participants at two distinct moments, researchers can analyze the outcomes. A high correlation coefficient, usually exceeding 0.80, signifies strong test-retest consistency, indicating that the test provides stable outcomes over time. A correlation coefficient of less than 0.70 may indicate poor reliability, while a coefficient of 0.90 or higher is considered excellent.
Inter-Rater Reliability: This assesses the degree of agreement between different raters or observers measuring the same phenomenon. It is particularly crucial in studies involving subjective judgments, ensuring that different researchers arrive at similar conclusions. For instance, studies in biopharma clinical trials often utilize Cohen's Kappa to quantify this agreement, with values above 0.70 considered acceptable. However, it is important to mention that Cohen's Kappa can be sensitive to the prevalence of agreement in the data, which may influence the understanding of outcomes.
Internal Consistency: This assesses the uniformity of outcomes across items within an assessment. Measured using Cronbach's alpha, a value above 0.70 indicates that the items are closely related and measure the same underlying construct. High internal consistency is essential for ensuring that the assessment accurately represents the intended concept.
Parallel-Forms Reliability: This involves comparing two different versions of an assessment that measure the same construct. By administering both forms to the same group, researchers can assess the consistency of results across different versions. This method is particularly useful in ensuring that variations in test administration do not affect outcomes.
Comprehending these categories of dependability is crucial for researchers striving to generate trustworthy and reproducible results, according to the scientific definition of reliability. High reliability not only reduces measurement error but also enhances the overall quality and credibility of the data, ultimately leading to more informed decision-making in research.
Reliability in scientific research is essential for ensuring consistent and stable measurements, which underpins the credibility of study results. This principle not only reinforces the validity of findings but also encourages collaboration among researchers, ultimately leading to more robust and reproducible studies. Highlighting the significance of reliable measurement tools is vital for advancing clinical research and achieving effective patient outcomes.
Key arguments emphasize various methods for assessing reliability, such as:
Each method is crucial in verifying that measurement tools produce trustworthy results, allowing researchers to build on previous findings and enhance the overall quality of data. The historical evolution of reliability measures, coupled with recent advancements in methodologies, reflects a steadfast commitment to improving research standards and outcomes.
In light of this discussion, it is evident that prioritizing reliability is not just an academic exercise; it is a critical component of effective scientific inquiry. Researchers must adopt rigorous reliability measures to strengthen the trustworthiness of their findings, thereby amplifying the impact of their work on healthcare practices and beyond. By committing to high standards of reliability, the scientific community can ensure that research translates into meaningful advancements and improved outcomes for all stakeholders involved.
What is the scientific definition of reliability in research?
Reliability in scientific research refers to the consistency and stability of measurements across time and contexts, indicating how well a measurement tool produces the same outcomes under stable conditions.
Why is reliability important in scientific research?
Reliability is important because it underpins the validity of study results, ensuring that findings can be reproduced and trusted.
How can reliability be assessed in scientific research?
Reliability can be assessed through various methods, including test-retest consistency, inter-rater consistency, and internal coherence.
What impact does understanding reliability have on clinical research outcomes?
Understanding and applying reliability measures can significantly enhance the credibility of findings and help researchers address key challenges in clinical research.
What role does reliability play in fostering collaboration among researchers?
Reliability supports the validity of results and fosters collaboration among researchers, paving the way for more robust and reproducible studies.
What should researchers prioritize in clinical research regarding measurement tools?
Researchers should prioritize the development and application of reliable measurement tools to enhance the quality of their studies.