


The Therapeutic Goods Administration (TGA) serves as Australia's regulatory authority, tasked with ensuring the safety, quality, and effectiveness of therapeutic goods, which encompass medications and medical devices. This article underscores the TGA's systematic evaluation processes, robust post-market surveillance, and proactive responsiveness to public health needs. Such functions illustrate the TGA's critical role in maintaining community trust and fostering innovation within the healthcare sector.
The Therapeutic Goods Administration (TGA) serves as a cornerstone of public health in Australia, ensuring that medications and medical devices meet stringent safety and efficacy standards. This regulatory body not only safeguards community health but also fosters innovation within the healthcare sector, providing vital oversight that benefits healthcare providers, patients, and manufacturers alike.
As the landscape of healthcare evolves, how does the TGA adapt its regulations to meet emerging challenges and opportunities? Exploring the multifaceted functions and historical evolution of the TGA reveals critical insights into its role in shaping a safer, more effective healthcare system.
The Therapeutic Goods Administration is Australia's regulatory authority responsible for ensuring the safety, quality, and effectiveness of therapeutic goods, which include medications, medical devices, and biological products. The Therapeutic Goods Administration was established under the Therapeutic Goods Act of 1989 and operates within the Australian Government's Department of Health. Its primary mission is to protect community health by enforcing rigorous criteria that medical products must meet before they can be marketed and utilized by individuals.
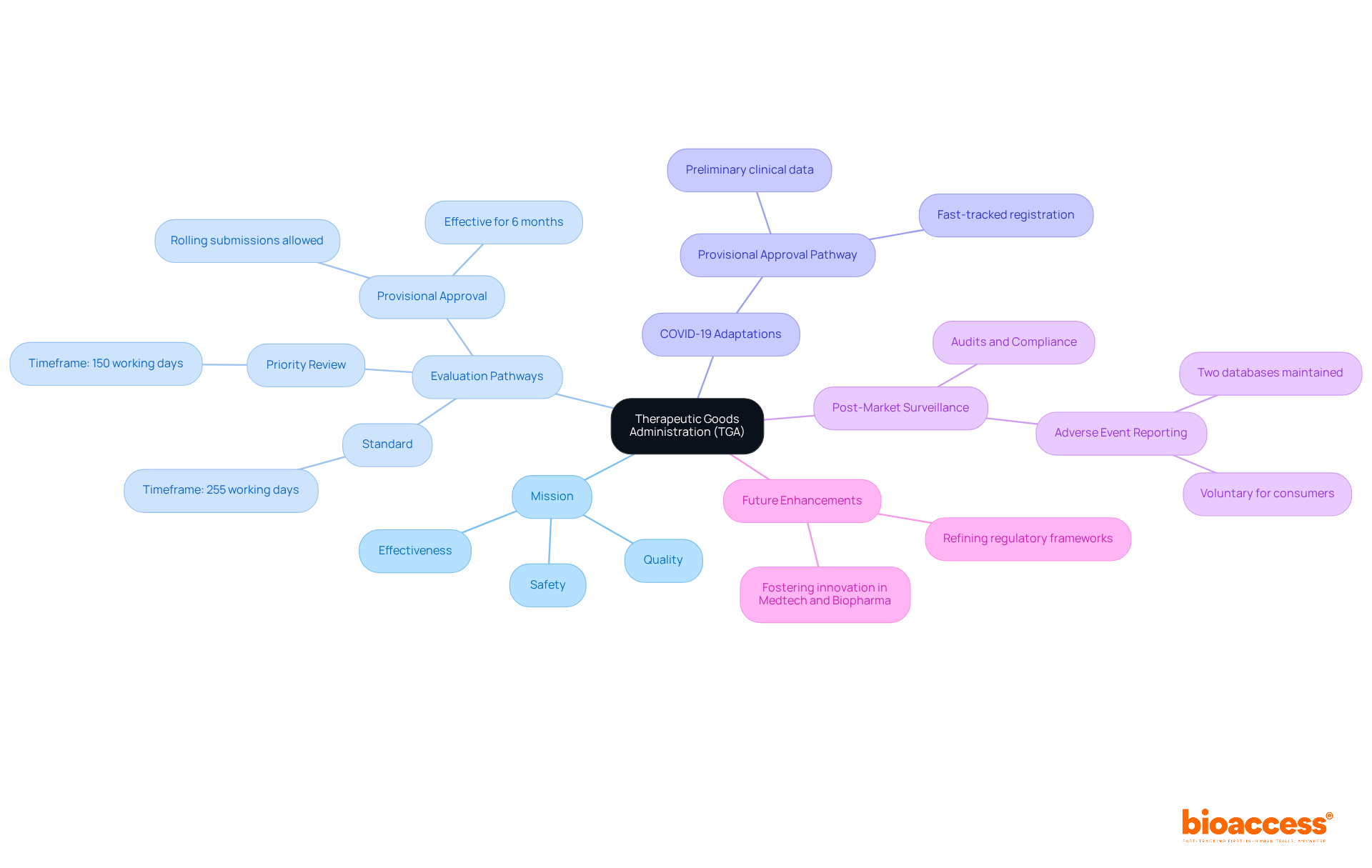
The TGA employs a systematic approach to evaluate therapeutic goods through various pathways, such as standard, priority review, and provisional approval, each designed to correspond with the associated risk levels of the products. For example, priority review accelerates the assessment of life-saving medicines within a target timeframe of 150 working days, whereas the standard pathway generally spans 255 working days. This methodical assessment process ensures that essential medications and devices are accessible to individuals while maintaining safety and effectiveness.
In recent years, the regulatory practices of the Therapeutic Goods Administration have been adapted to meet public health emergencies, particularly during the COVID-19 pandemic. The Therapeutic Goods Administration leveraged the provisional approval pathway to expedite the registration of vaccines and treatments, showcasing its responsiveness to urgent health needs. This pathway allows for provisional registration based on preliminary clinical data, with the opportunity for ongoing submissions for additional information, thereby facilitating quicker access to vital medical products.
The Therapeutic Goods Administration's influence extends beyond initial approvals; it also plays a pivotal role in post-market surveillance. By maintaining databases for adverse event reporting and conducting audits, the TGA ensures the continuous safety and compliance of therapeutic goods in the marketplace. This vigilant oversight is crucial for fostering community trust in the healthcare system, as it promptly addresses safety concerns through alerts and recalls when necessary.
As of 2025, the Therapeutic Goods Administration (TGA) continues to enhance its regulatory authority by refining its frameworks to better serve the Australian community and the healthcare sector. By prioritizing the safety and effectiveness of medical products, the Therapeutic Goods Administration not only protects public health but also fosters innovation within the Medtech and Biopharma sectors, ultimately contributing to improved health outcomes nationwide. Companies like bioaccess® can assist Medtech, Biopharma, and Radiopharma startups in navigating these regulatory pathways, enabling them to initiate clinical trials 40% faster, secure regulatory approvals, and enhance patient recruitment efforts while connecting with top-tier clinical research sites.
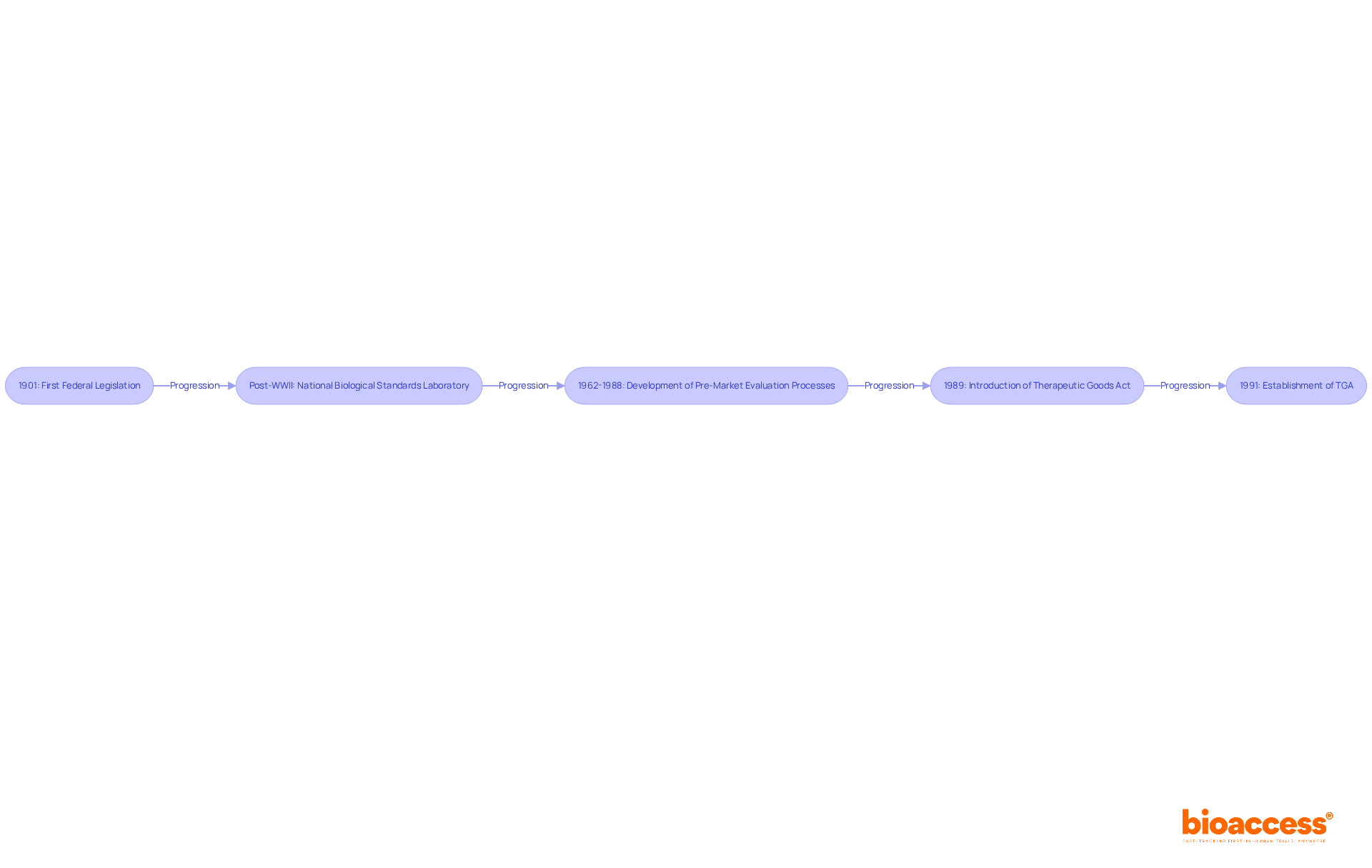
The origins of the therapeutic goods administration (TGA) date back to the early 1900s, driven by an increasing demand for oversight of health-related products. In 1901, the first federal legislation aimed at ensuring the quality of medicines was enacted, marking the inception of a structured approach to medicine regulation in Australia. Over the years, this regulatory framework has undergone significant transformations, reflecting advancements in medical science and the complexities of healthcare delivery.
A pivotal moment in this evolution was the introduction of the Therapeutic Goods Act of 1989, which led to the establishment of the therapeutic goods administration in 1991. This act unified various regulatory functions, creating a comprehensive system for the evaluation and oversight of health products. The establishment of the therapeutic goods administration (TGA) was a direct response to the growing necessity for stringent supervision in a rapidly evolving healthcare landscape, ensuring that medical products meet high standards of quality, safety, and efficacy.
Key milestones in the TGA's history include:
Today, the therapeutic goods administration (TGA) is recognized as a respected global authority, ensuring the safety and effectiveness of medical products in Australia. Its regulatory framework continues to evolve, addressing the challenges of modern healthcare while reflecting a mature and harmonized system that prioritizes public health.
The therapeutic goods administration plays a pivotal role in ensuring that therapeutic products are not only safe but also effective and of high quality. Central to the mission of the therapeutic goods administration is the regulation of:
of these goods. The therapeutic goods administration (TGA) performs rigorous assessments of new medicines and medical devices before they enter the market, ensuring compliance with stringent safety and efficacy standards. In 2025, the therapeutic goods administration updated its safety and effectiveness quality standards, highlighting its unwavering commitment to community health. Additionally, the therapeutic goods administration's post-market monitoring is crucial for tracking the ongoing safety and effectiveness of products, which facilitates timely interventions when necessary. This vigilant oversight is essential for maintaining community trust in the healthcare system, as demonstrated by the therapeutic goods administration's proactive measures in addressing emerging safety concerns.
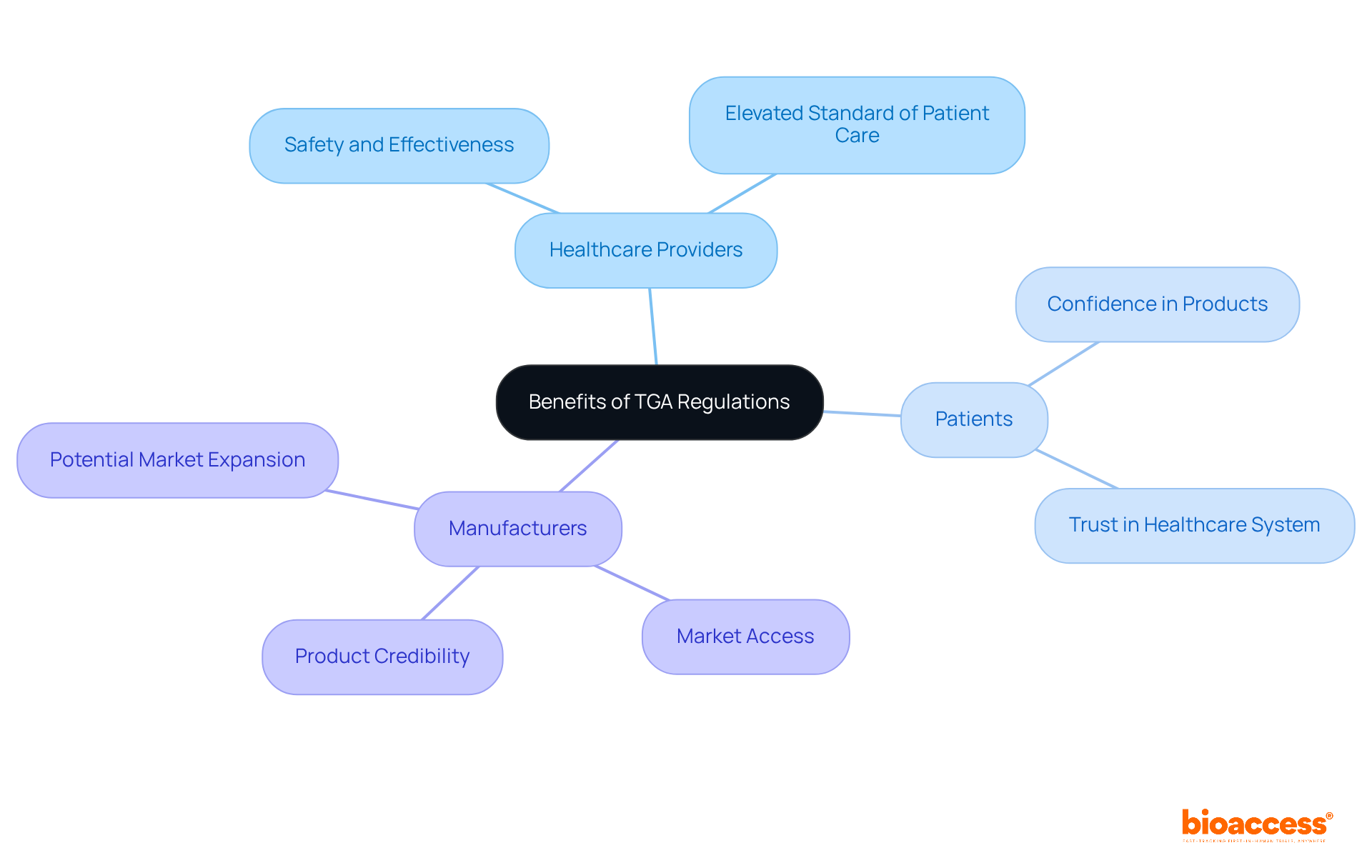
The regulations enforced by the therapeutic goods administration yield significant advantages for healthcare providers, patients, and manufacturers alike. For healthcare providers, the therapeutic goods administration regulations guarantee that the therapeutic goods prescribed are both safe and effective, thereby elevating the standard of patient care. Patients gain confidence knowing that the products they utilize have undergone thorough evaluations, fostering trust in the healthcare system. For manufacturers, adherence to the therapeutic goods administration regulations not only facilitates market access but also bolsters product credibility, leading to heightened consumer confidence and potential market expansion.
In 2025, the therapeutic goods administration's commitment to rigorous compliance is expected to further enhance market access, as manufacturers increasingly recognize the value of regulatory endorsement in establishing their products. Notably, mandatory reporting of medical device-related injuries will begin on 21 March 2026, reflecting the therapeutic goods administration's commitment to improving safety measures. Furthermore, bioaccess offers comprehensive clinical trial management services, including:
which are essential for navigating these regulations effectively.
By upholding high standards, the therapeutic goods administration plays a crucial role in promoting innovation while safeguarding public health, ultimately benefiting all stakeholders involved.
The Therapeutic Goods Administration (TGA) stands as a pivotal element in safeguarding public health in Australia, ensuring that therapeutic goods, including medications and medical devices, are not only safe and effective but also of the highest quality. Through the implementation of rigorous regulatory frameworks and an ability to adapt to urgent health needs, the TGA not only protects community health but also promotes innovation within the healthcare sector.
Historically, the TGA has undergone significant evolution, addressing the complexities of modern healthcare while steadfastly upholding its commitment to safety and efficacy. Its key functions—such as pre-market assessments and post-market surveillance—exemplify a comprehensive regulatory approach. The advantages of these regulations extend beyond mere compliance; they cultivate trust among patients and healthcare providers, while simultaneously empowering manufacturers to confidently access markets.
Given the TGA's critical role, it is imperative for all stakeholders—healthcare providers, patients, and manufacturers—to acknowledge and support its initiatives. By grasping the significance of TGA regulations, stakeholders can actively contribute to a healthier future, ensuring that therapeutic goods consistently meet the highest standards of safety and effectiveness. Engaging with the TGA and remaining informed about its evolving regulations can ultimately lead to enhanced health outcomes for all Australians.
What is the Therapeutic Goods Administration (TGA)?
The Therapeutic Goods Administration (TGA) is Australia's regulatory authority responsible for ensuring the safety, quality, and effectiveness of therapeutic goods, including medications, medical devices, and biological products.
When was the TGA established?
The TGA was established under the Therapeutic Goods Act of 1989.
What is the primary mission of the TGA?
The primary mission of the TGA is to protect community health by enforcing rigorous criteria that medical products must meet before they can be marketed and used by individuals.
How does the TGA evaluate therapeutic goods?
The TGA employs a systematic approach to evaluate therapeutic goods through various pathways, such as standard, priority review, and provisional approval, which correspond to the associated risk levels of the products.
What is the difference between the priority review and standard pathways?
The priority review pathway accelerates the assessment of life-saving medicines within a target timeframe of 150 working days, while the standard pathway generally spans 255 working days.
How did the TGA respond to public health emergencies like COVID-19?
The TGA adapted its regulatory practices during the COVID-19 pandemic by leveraging the provisional approval pathway to expedite the registration of vaccines and treatments based on preliminary clinical data.
What role does the TGA play in post-market surveillance?
The TGA is involved in post-market surveillance by maintaining databases for adverse event reporting and conducting audits to ensure the continuous safety and compliance of therapeutic goods in the marketplace.
How does the TGA contribute to community trust in the healthcare system?
The TGA fosters community trust by promptly addressing safety concerns through alerts and recalls when necessary, ensuring the ongoing safety of therapeutic goods.
What are the future plans for the TGA as of 2025?
As of 2025, the TGA plans to enhance its regulatory authority by refining its frameworks to better serve the Australian community and the healthcare sector, prioritizing the safety and effectiveness of medical products.
How can companies like bioaccess® assist startups in the Medtech and Biopharma sectors?
Companies like bioaccess® can help Medtech, Biopharma, and Radiopharma startups navigate regulatory pathways, enabling them to initiate clinical trials 40% faster, secure regulatory approvals, and enhance patient recruitment efforts while connecting with top-tier clinical research sites.