


The article delineates the definition and classification of medical devices in accordance with US FDA standards, underscoring their intended use, technological characteristics, and regulatory requirements. It elucidates that medical devices are classified into three categories based on risk—
with each category possessing distinct approval processes that influence clinical research and market strategies. This classification is pivotal in ensuring adherence to safety and effectiveness standards, thereby reinforcing the importance of compliance in the Medtech landscape.
Understanding the intricate landscape of medical devices is crucial for anyone involved in healthcare innovation. The US FDA defines a medical device not solely by its function but also by its classification, which significantly impacts safety, effectiveness, and market access. As the regulatory environment evolves, navigating the complexities of device classifications—Class I, II, and III—presents both challenges and opportunities for manufacturers.
How can stakeholders effectively maneuver through these classifications to ensure compliance and successful market entry while addressing the unique risks associated with each category?
According to the us fda medical device definition, a healthcare instrument is any tool, machine, contrivance, implant, in vitro reagent, or related article intended for use in diagnosing, curing, mitigating, treating, or preventing disease. Key characteristics of medical devices include:
Regulatory compliance means that all equipment must adhere to the us fda medical device definition to guarantee safety and effectiveness. For example, Category I items, such as sterile bandages and manual wheelchairs, experience minimal regulatory control, while Category II items, including X-ray systems and contact lenses, necessitate more stringent regulations. Category III products, such as implantable defibrillators and heart valves, require strict Premarket Approval (PMA) because of their high-risk nature.
In 2025, the FDA's classification system still organizes over 1,700 types of healthcare instruments into three categories based on risk: Class I (low risk), Class II (moderate risk), and Class III (high risk). This categorization is essential for identifying the pathway and requirements for promoting a product. Grasping these standards is crucial for producers seeking to maneuver through the intricate terrain of healthcare product approval. Furthermore, authorizations for healthcare equipment increased by 15% in relation to the prior quarter, showcasing the dynamic characteristics of the market. According to the us fda medical device definition, a product designed to influence the structure or function of a human or animal's body can be categorized as a healthcare instrument. This understanding is vital for ensuring compliance and successful market entry.
To facilitate this process, bioaccess® offers comprehensive clinical trial management services tailored to the needs of Medtech, Biopharma, and Radiopharma startups. These services include:
With more than 20 years of experience in overseeing Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies, bioaccess® is well-prepared to assist startups in maneuvering through the compliance environment efficiently.
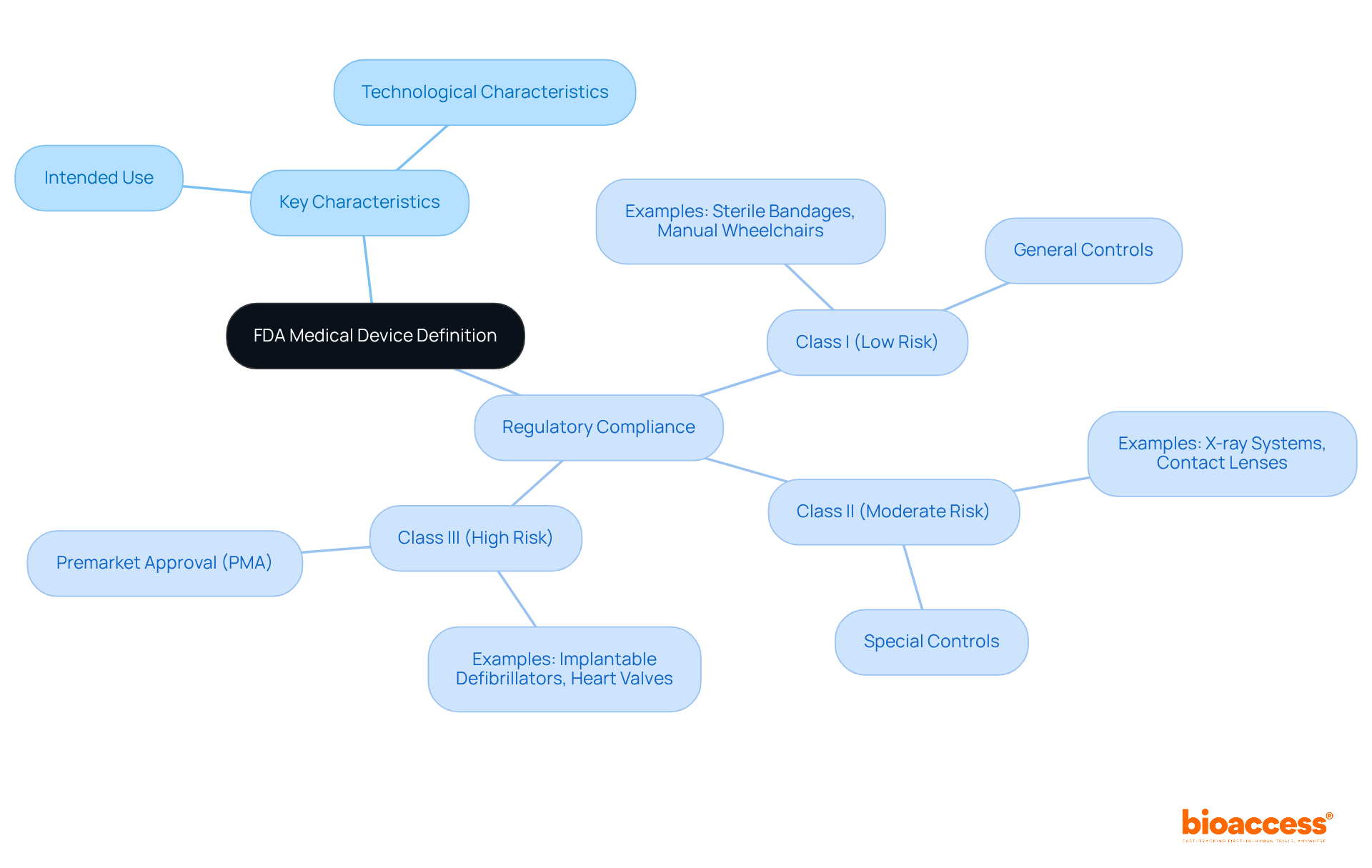
The FDA categorizes medical devices into three classes based on their associated risk levels:
Class I: These are low-risk devices that face minimal regulatory control. Common examples include bandages, tongue depressors, and manual stethoscopes. Approximately 95% of Class I products are exempt from premarket notification, reflecting their established safety profile.
Class II: Representing moderate-risk products, Class II requires more stringent regulatory oversight to ensure safety and effectiveness. Examples include infusion pumps, pregnancy test kits, and surgical gloves. Most Class II products require a 510(k) premarket notification to demonstrate substantial equivalence to current products.
Class III: High-risk items belong to this group, generally sustaining or supporting life, being implanted, or presenting a significant risk of illness or injury. Examples include pacemakers, breast implants, and high-frequency ventilators. Class III products must go through the Premarket Approval (PMA) process, necessitating extensive clinical data to confirm their safety and effectiveness.
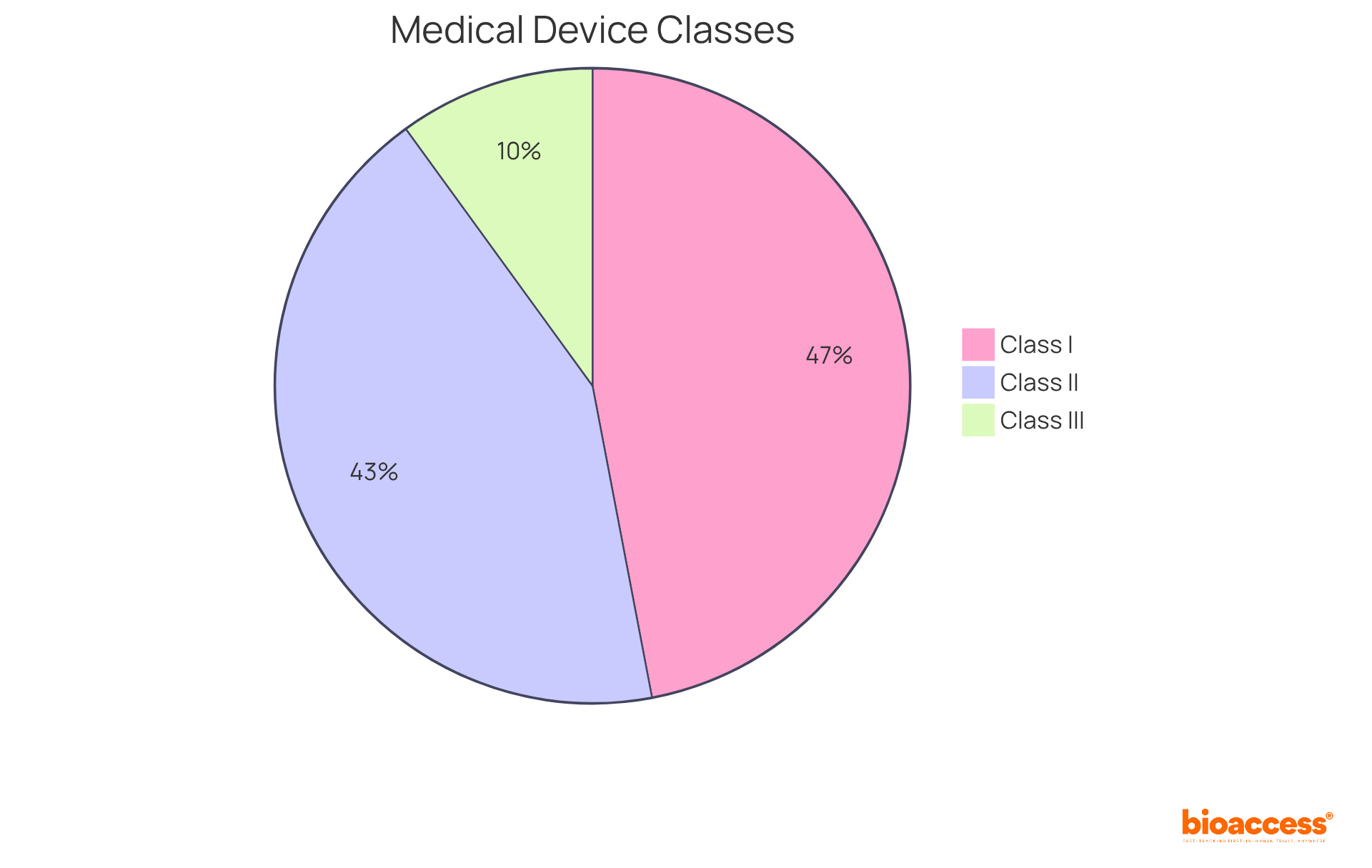
According to recent reports, roughly 47% of healthcare instruments are categorized as Class I, 43% as Class II, and around 10% as Class III. This classification system is essential for guaranteeing that equipment is properly managed according to the US FDA medical device definition and their potential hazards to patients. Specialists underscore the significance of grasping these classifications, as they directly influence the compliance routes, market entry for healthcare innovations, and the US FDA medical device definition.
In Colombia, the regulatory environment is supervised by INVIMA (Instituto Nacional de Vigilancia de Medicamentos y Alimentos), which plays a crucial role in ensuring the safety, efficacy, and quality of healthcare instruments. Established in 1992 under the Ministry of Health and Social Protection, INVIMA is responsible for inspecting and supervising the marketing and manufacturing of health products. The Directorate for Health Products and other Technologies within INVIMA oversees and regulates health products, proposing technical standards for their production and quality assurance. Acknowledged as a Level 4 health authority by the Pan American Health Organization/World Health Organization, INVIMA's stringent supervision parallels the FDA's classification system, ensuring that health products in Colombia meet high safety and efficacy standards. Katherine Ruiz, an expert in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, emphasizes the importance of understanding these regulatory frameworks for successful market access and compliance.
The approval processes for medical devices are categorized by their classification, each with distinct requirements and timelines:
Class I: Most Class I devices, which include low-risk items like tongue depressors and electric toothbrushes, are exempt from the premarket notification process. However, manufacturers are still required to register their establishment and list their products with the FDA. The self-registration process is typically the fastest path to market, often completed within a week, although it may take several days for fee acceptance before electronic submission.
Class II: For Class II products, which encompass moderate-risk items such as contact lenses and syringes, manufacturers must submit a 510(k) premarket notification. This submission demonstrates that the apparatus is significantly comparable to a legally marketed product. The average time for clearance under the traditional 510(k) pathway is approximately 177 days, with only 19% of submissions cleared within three months. In the year leading up to September 2022, 85% of 510(k) applications received a Substantially Equivalent decision, indicating regulatory approval for market sale in the US.
Class III: Class III products, which encompass high-risk items like cochlear implants and defibrillators, necessitate a more rigorous Premarket Approval (PMA) application. This process requires substantial clinical data to support the safety and effectiveness of the apparatus. The average approval time for PMA applications has improved significantly, with the FDA reporting an average decision time of 209 days in recent years. However, the intricacy of the apparatus and the data needed can prolong this timeline to several months or even years.
Grasping these regulatory pathways is essential for producers as they navigate the intricacies of product approval, ensuring prompt market entry and compliance with the US FDA medical device definition.

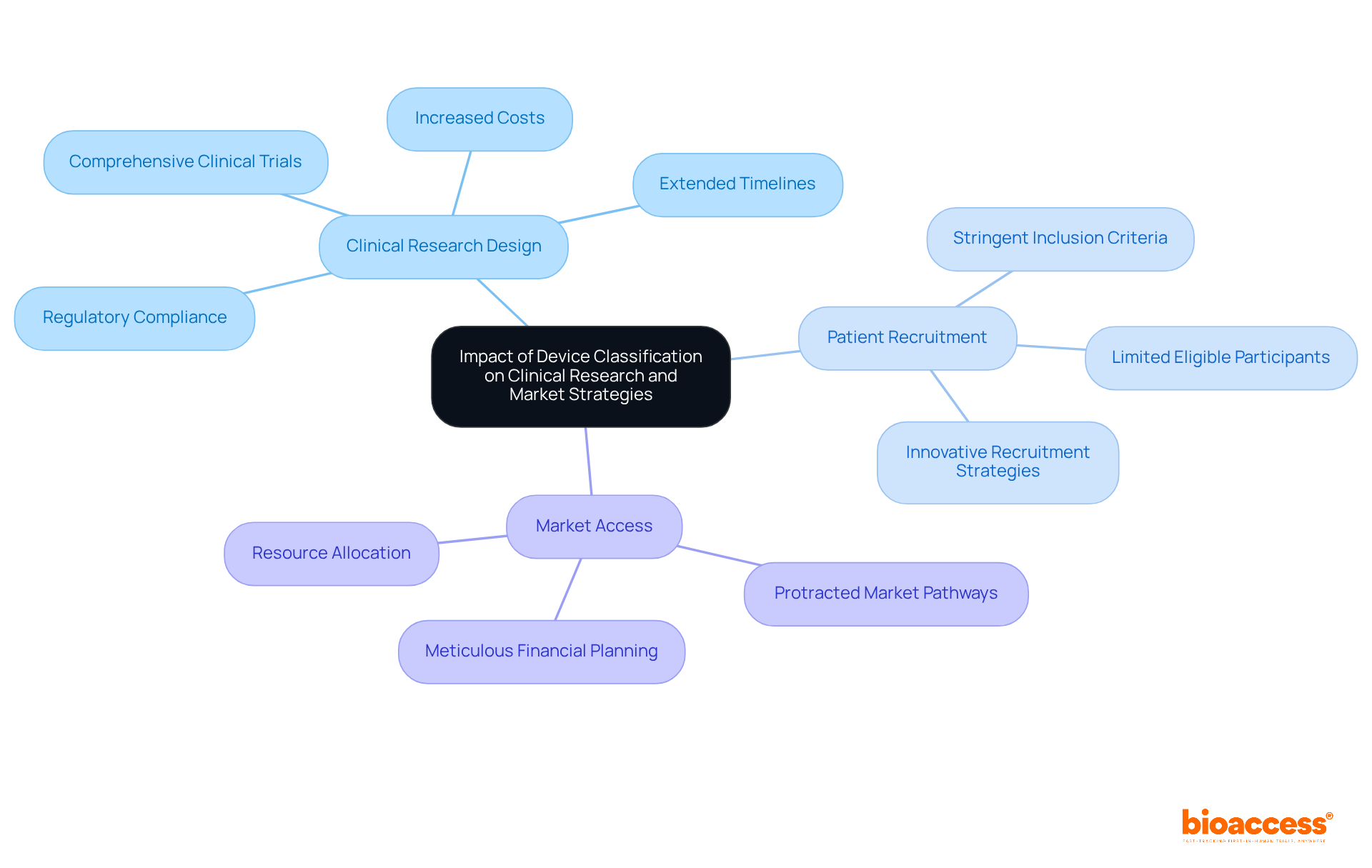
The classification of equipment significantly influences various aspects of clinical studies and market strategies, particularly concerning Category III medical instruments, which are deemed higher-risk.
Clinical Research Design: Class III products require comprehensive clinical trials to establish safety and efficacy, often resulting in extended timelines and increased costs. A meticulously structured study design is essential, as it must comply with the stringent requirements imposed by regulatory authorities. For instance, the FDA mandates that studies for these products provide exhaustive data on safety and effectiveness, complicating the design process.
Patient Recruitment: The classification directly affects patient recruitment methodologies. Level III instruments typically demand more stringent inclusion criteria, which can limit the pool of eligible participants. This necessitates innovative recruitment strategies to ensure that trials are sufficiently powered and representative of the target population. Tailored outreach efforts may be required to engage specific patient demographics that fulfill the higher-risk criteria.
Market Access: The pathway to market for Level III products is often more protracted than that for Levels I and II products, which may benefit from expedited approval processes. This prolonged timeline necessitates meticulous financial planning and adjustments to market strategies. Businesses must anticipate potential setbacks and allocate resources effectively to navigate the complexities of approval processes.
Current trends in clinical research design for Class III devices highlight the significance of adaptive trial methodologies and real-world evidence to enhance patient engagement and streamline the approval process. As the market continues to evolve, leveraging innovative strategies will be crucial for successfully navigating the regulatory landscape.
Understanding the US FDA medical device definition and its classifications is crucial for anyone involved in the healthcare industry. Medical devices, as defined by the FDA, encompass a wide range of instruments designed for medical purposes. Their classification into Class I, II, and III directly impacts regulatory requirements and market strategies.
This article provided key insights into the characteristics that define medical devices, the regulatory pathways for each class, and the implications of these classifications on clinical research and market access.
Understanding these distinctions is vital for manufacturers and stakeholders aiming to navigate the complex regulatory landscape effectively.
In a rapidly evolving healthcare market, staying informed about FDA classifications and their implications is essential for ensuring compliance and successful product launches. Embracing innovative strategies and thorough planning can facilitate a smoother path to market, especially for high-risk devices. As the medical device landscape continues to change, leveraging insights from this classification system will empower stakeholders to make informed decisions that ultimately enhance patient safety and drive advancements in healthcare technology.
What is the FDA definition of a medical device?
According to the FDA, a medical device is any tool, machine, contrivance, implant, in vitro reagent, or related article intended for use in diagnosing, curing, mitigating, treating, or preventing disease.
What are the key characteristics of medical devices?
The key characteristics of medical devices include intended use, which is the specific medical purpose for which the device is designed, and technological characteristics, which encompass the design, components, and operational mechanisms of the device.
How does regulatory compliance work for medical devices?
Regulatory compliance means that all medical devices must adhere to the FDA's definition to ensure safety and effectiveness. The FDA classifies devices into three categories based on risk: Class I (low risk), Class II (moderate risk), and Class III (high risk), with varying levels of regulatory control.
What are examples of different categories of medical devices?
Category I items, such as sterile bandages and manual wheelchairs, experience minimal regulatory control. Category II items, like X-ray systems and contact lenses, require more stringent regulations. Category III products, such as implantable defibrillators and heart valves, necessitate strict Premarket Approval (PMA).
How many types of healthcare instruments does the FDA classify, and what is the significance of this classification?
The FDA classifies over 1,700 types of healthcare instruments into three categories based on risk. This classification is essential for identifying the pathway and requirements for promoting a product in the market.
What services does bioaccess® offer to assist startups in the medical device field?
Bioaccess® offers comprehensive clinical trial management services, including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting.
What experience does bioaccess® have in managing clinical trials?
Bioaccess® has more than 20 years of experience in overseeing Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies, making them well-prepared to assist startups in navigating the compliance environment effectively.