

The Tanzania Medicines and Medical Devices Authority (TMDA) serves a pivotal role in regulating the safety, effectiveness, and quality of healthcare products in Tanzania. This authority is essential not only for ensuring public health but also for fostering trust within the community and encouraging investment in the healthcare sector.
TMDA's functions include:
These activities collectively enhance the reliability of healthcare products available to the public, addressing key challenges in the Medtech landscape. By reinforcing the importance of safety and efficacy, TMDA positions itself as a cornerstone of the healthcare system, prompting stakeholders to engage further in clinical research and product development.
The Tanzania Medicines and Medical Devices Authority (TMDA) stands as a pivotal guardian of public health, ensuring that the medicines and healthcare devices accessible to Tanzanians adhere to stringent safety and quality standards.
In a rapidly evolving healthcare landscape, comprehending the multifaceted role of TMDA is crucial for stakeholders, including pharmaceutical companies and healthcare providers.
With the alarming rise of counterfeit products and an urgent demand for innovation, how adeptly can TMDA navigate these challenges to fulfill its mission?
This article explores the authority's historical evolution, regulatory framework, and significant influence on healthcare in Tanzania, illuminating the essential balance between safety and advancement in medical regulation.
The tmda is the governing entity responsible for ensuring the safety, effectiveness, and quality of medicines and healthcare devices in Tanzania. Established under the Medicines and Medical Devices Act of 2003, this agency is dedicated to safeguarding public health by ensuring that all healthcare products meet stringent safety and quality criteria before they become available to the public. This authority is pivotal in the oversight environment, functioning as a gatekeeper for healthcare innovations and ensuring compliance with both national and international standards.
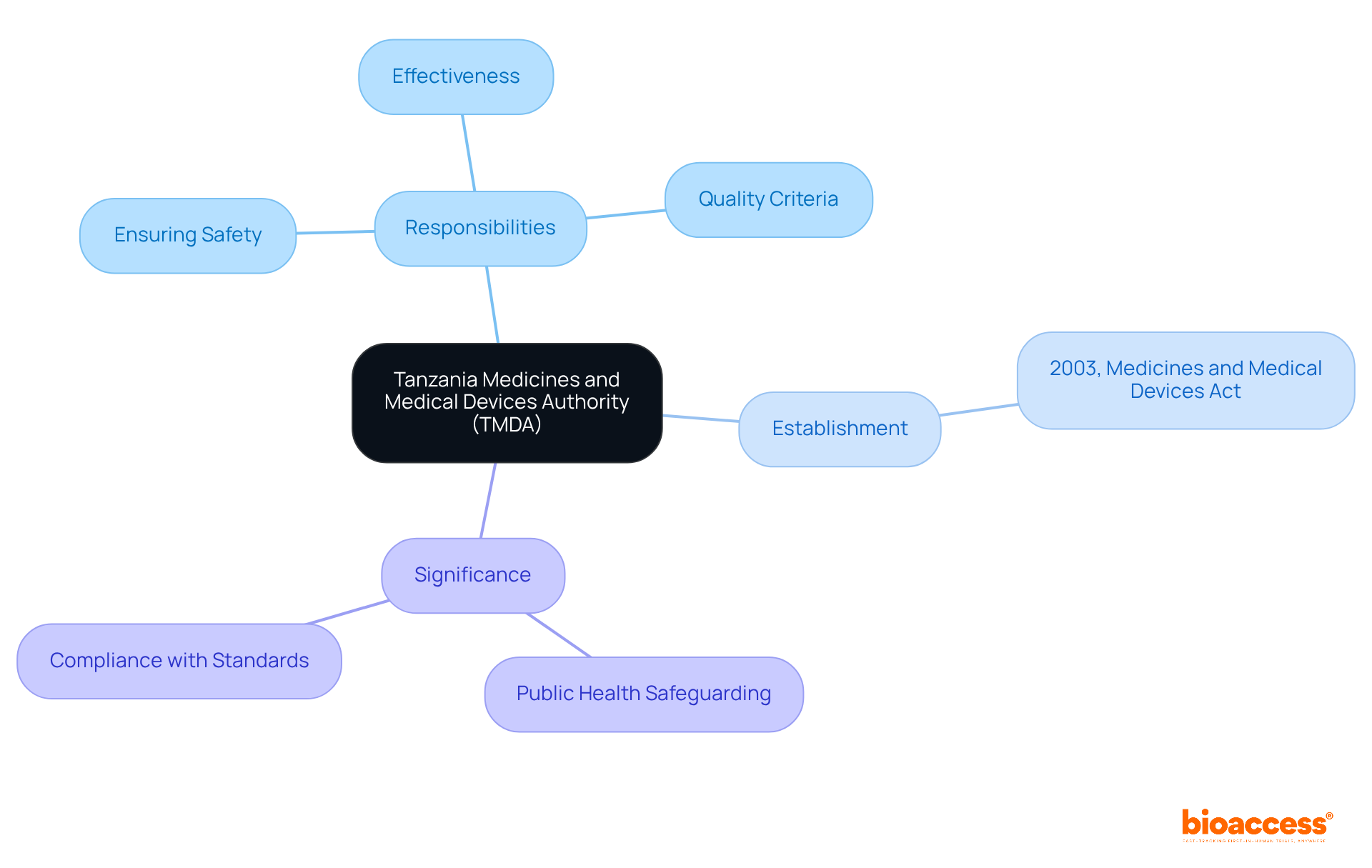
The organization was established to meet the growing demand for a robust oversight structure for the introduction of healthcare products in Tanzania. Operating under the Ministry of Health, Community Development, Gender, Elderly and Children, it adheres to a regulatory framework shaped by various laws and guidelines, including:
Over the years, the organization has adapted its regulations to address emerging health challenges, such as the rise of counterfeit drugs and the urgent need for expedited approval processes for innovative health technologies.
Understanding the regulations set forth by the relevant authority is crucial for firms like bioaccess, which provide clinical trial management services. Compliance with these guidelines ensures that clinical trials conducted in Tanzania meet essential standards, thereby facilitating smoother approval processes and enhancing the overall success of device studies. As the Medtech landscape evolves, collaboration with regulatory bodies becomes increasingly vital, positioning firms to effectively navigate the complexities of clinical research and drive advancements in healthcare.
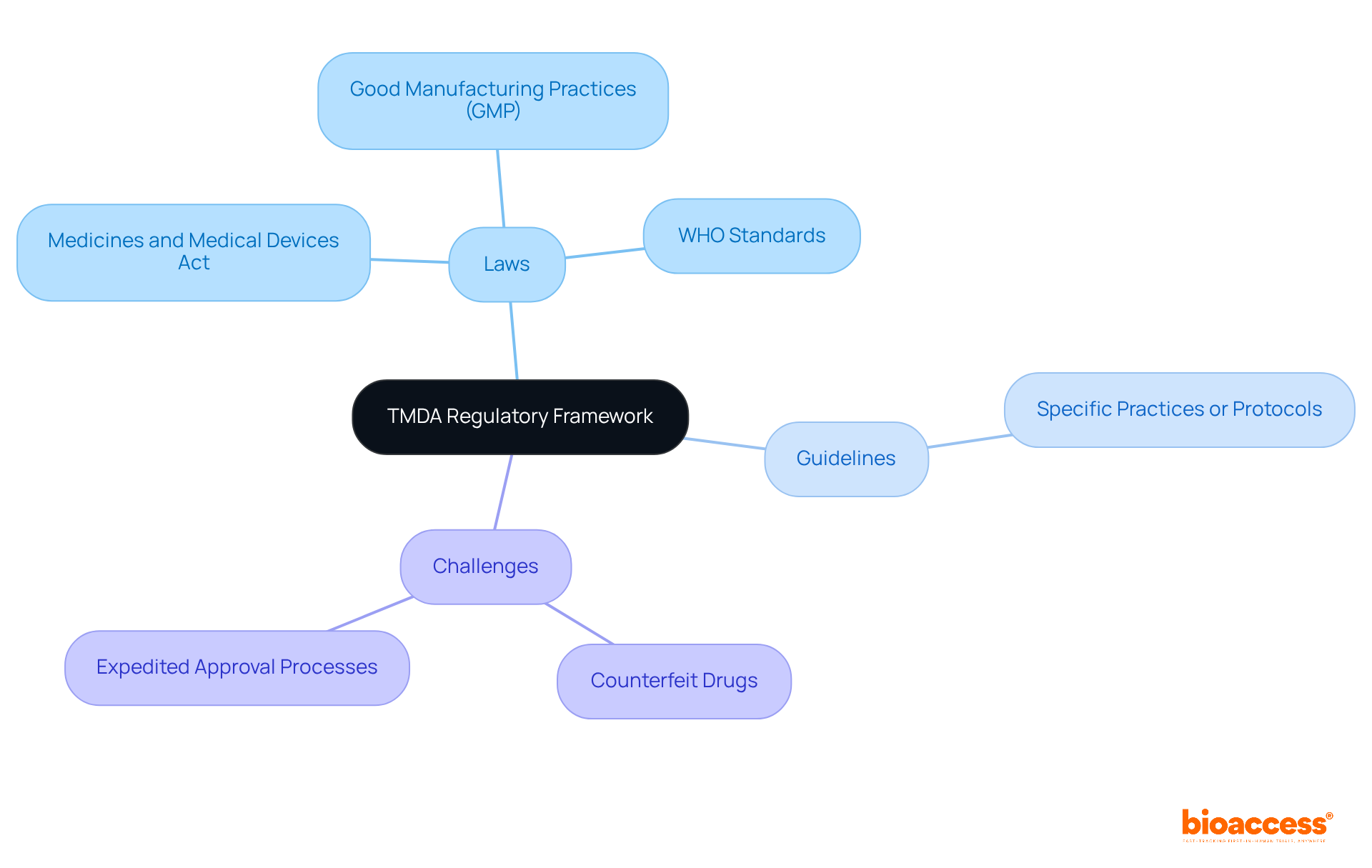
The organization's primary functions encompass the assessment and registration of pharmaceuticals and healthcare devices, overseeing their safety and effectiveness post-market introduction, and ensuring adherence to regulatory standards. The authority conducts inspections of manufacturing facilities to guarantee compliance with Good Manufacturing Practices (GMP) and oversees clinical trials to protect the rights and welfare of participants. Furthermore, the organization plays a crucial role in public health education, informing healthcare professionals and the public about the safe use of medical products. By fulfilling these functions, tmda ensures that only safe and effective products reach the Tanzanian market.
With bioaccess®'s comprehensive clinical trial management services, the process is streamlined, allowing for accelerated regulatory approval. These services encompass:
Bioaccess®'s innovative 6-8 week sprint approach enables faster enrollment of treatment-naive cardiology and neurology cohorts, significantly impacting local economies through job creation and healthcare improvement. This collaboration not only enhances the efficiency of clinical trials but also promotes international partnerships, ultimately aiding health on a broader scale.

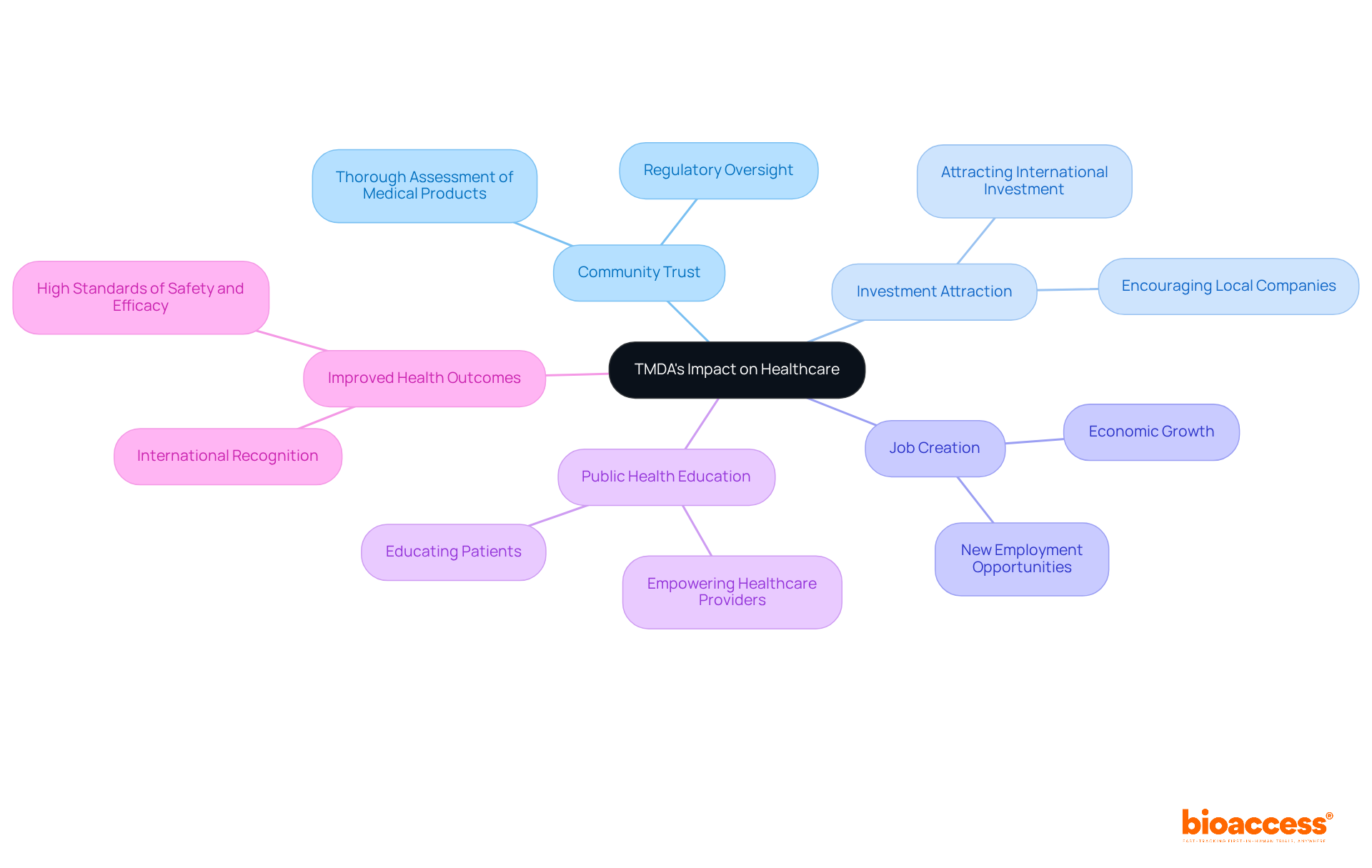
The influence of the organization on healthcare in Tanzania is profound. By ensuring that medical products are thoroughly assessed and overseen, the agency enhances community trust in healthcare systems and promotes an atmosphere favorable to innovation. This regulatory oversight encourages both local and international companies to invest in the Tanzanian market, creating jobs and promoting economic growth.
Furthermore, the organization's dedication to public health education empowers healthcare providers and patients, resulting in improved health outcomes. Stakeholders, including pharmaceutical companies, healthcare professionals, and patients, benefit from TMDA's efforts to maintain high standards of safety and efficacy in medical products, which also contributes to TMDA's international recognition.
Ultimately, these initiatives contribute to the overall improvement of healthcare delivery in Tanzania while driving global health improvement through international collaboration and innovation in Medtech.
The Tanzania Medicines and Medical Devices Authority (TMDA) serves as a vital cornerstone in the nation's healthcare framework, committed to ensuring that medicines and medical devices are not only safe and effective but also of the highest quality. Established under the Medicines and Medical Devices Act of 2003, TMDA is instrumental in safeguarding public health while fostering innovation within the healthcare sector.
With a robust regulatory framework, TMDA diligently assesses and registers pharmaceuticals and healthcare devices, overseeing their safety and efficacy even after they reach the market. Its unwavering dedication to Good Manufacturing Practices and adherence to international standards cultivates community trust and stimulates investment in Tanzanian healthcare. By actively engaging with stakeholders, including pharmaceutical firms and healthcare professionals, TMDA enhances the overall quality of healthcare delivery and propels economic growth.
Given TMDA's significant contributions, it is essential for stakeholders to engage proactively with this regulatory authority. The collaboration between TMDA and healthcare innovators is crucial for effectively navigating the complexities of medical product regulation and advancing public health initiatives. By emphasizing compliance and nurturing a culture of safety, TMDA's impact will resonate throughout Tanzania, ultimately leading to improved health outcomes and a more resilient healthcare system.
What is the TMDA?
The TMDA, or Tanzania Medicines and Medical Devices Authority, is the governing entity responsible for ensuring the safety, effectiveness, and quality of medicines and healthcare devices in Tanzania.
When was the TMDA established?
The TMDA was established under the Medicines and Medical Devices Act of 2003.
What is the primary role of the TMDA?
The primary role of the TMDA is to safeguard public health by ensuring that all healthcare products meet stringent safety and quality criteria before they are made available to the public.
How does the TMDA contribute to public health?
The TMDA contributes to public health by acting as a gatekeeper for healthcare innovations and ensuring compliance with both national and international standards for medicines and medical devices.