

The article emphasizes the definitions, classifications, and significance of biomedical devices in healthcare, clearly stating their crucial roles in diagnosis, treatment, and patient monitoring. It builds interest by presenting compelling market growth statistics and noteworthy examples, such as continuous glucose monitors and MRI machines. These devices not only enhance clinical decision-making but also significantly improve patient outcomes, reinforcing the conviction that their integration is essential in modern healthcare. The discussion prompts readers to consider the ongoing advancements and the collaborative efforts required to address key challenges in the Medtech landscape.
Biomedical devices are integral to modern healthcare, fundamentally transforming the diagnosis, treatment, and monitoring of diseases. Ranging from basic instruments like tongue depressors to sophisticated technologies such as MRI machines and implantable devices, these tools significantly enhance clinical precision and improve patient outcomes.
As the biomedical technology landscape evolves, it becomes crucial to understand the diverse types of devices, their classifications, and the regulatory frameworks that govern their use. This raises essential questions about safety, effectiveness, and the future of patient care.
What implications do these advancements hold for healthcare providers and patients alike?
Biomedical instruments include many types of biomedical devices, such as tools, machines, implants, and software specifically designed for medical applications, including the diagnosis, prevention, monitoring, and treatment of diseases. These instruments encompass different types of biomedical devices, ranging from basic tools like tongue depressors to sophisticated systems such as pacemakers and MRI machines. Their significance is underscored by their ability to enhance diagnostic precision, which is crucial for delivering efficient care. For instance, the in-vitro diagnostics market was valued at $91.2 billion in 2022 and is anticipated to grow to $118 billion by 2030, reflecting an increasing reliance on diagnostic tools that bolster clinical decision-making.
The functionality of types of biomedical devices in clinical practice is essential. They not only streamline workflows but also equip healthcare professionals with critical data that informs treatment strategies. Real-world examples of types of biomedical devices include continuous glucose monitoring systems like the newly launched Dexcom G7, which improves diabetes management through enhanced accuracy and convenience by offering real-time insights into blood sugar levels and thereby elevating patient outcomes. Moreover, advancements in imaging technologies, including MRI and CT scans, have markedly improved diagnostic precision in the types of biomedical devices, facilitating earlier detection of conditions like cancer, which impacts over 1.7 million Americans annually. The sector of diagnostic imaging equipment, as one of the types of biomedical devices, saw a 6.2% increase in revenue, emphasizing its vital role in modern healthcare.
Healthcare experts recognize the transformative potential of these instruments. As Dr. Glen Stream remarked, "We believe consumer health technologies — apps, wearables, self-diagnosis tools — have the potential to strengthen the patient-physician connection and improve health outcomes." By understanding the diverse functions of biomedical instruments, healthcare providers can effectively leverage these tools to optimize treatment pathways and enhance overall individual well-being.
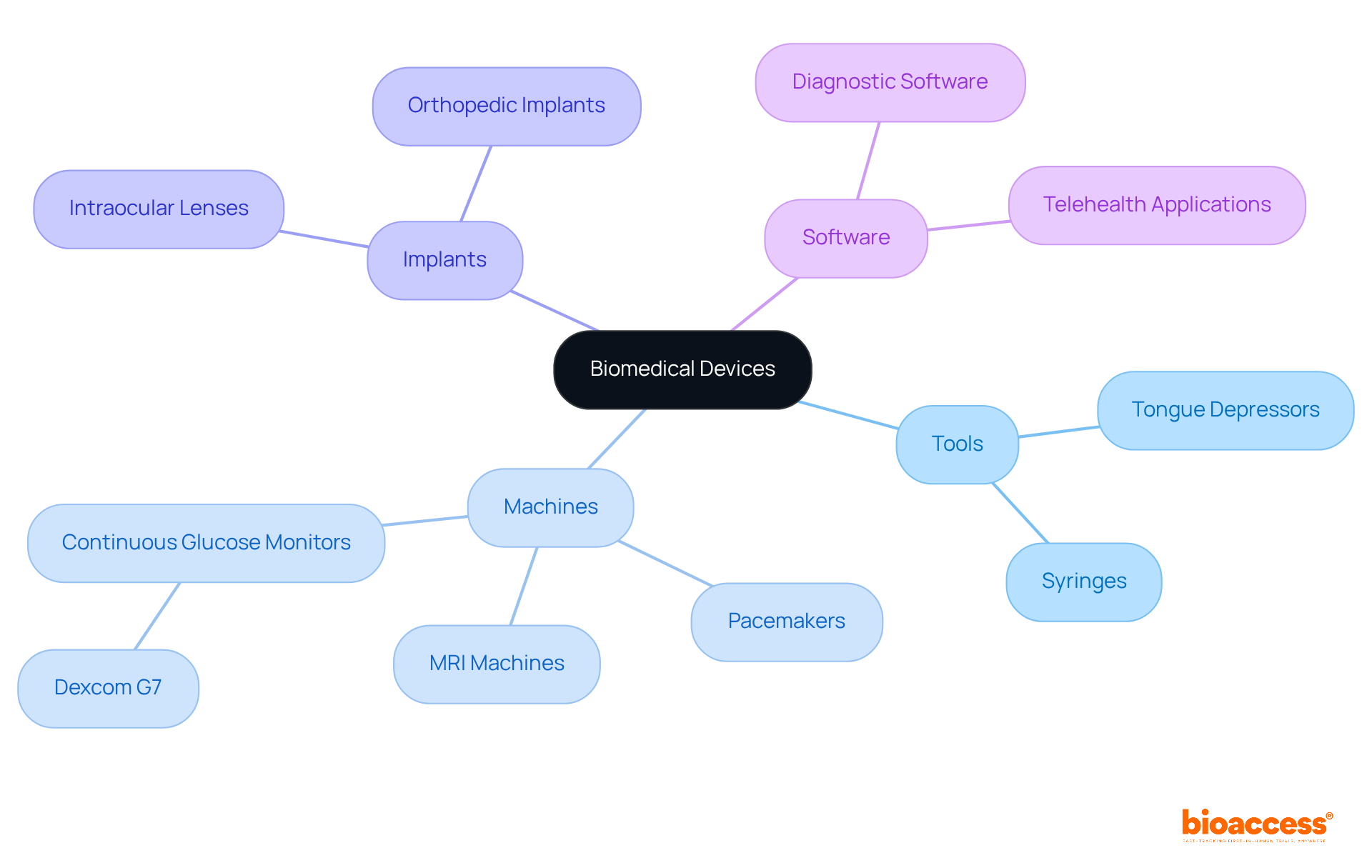
The categorization of types of biomedical devices is based on their intended use and associated risk levels, which is crucial for effective patient care. The primary classifications include:
Diagnostic Devices: Essential for identifying medical conditions, these tools encompass MRI machines, blood glucose monitors, and in-vitro diagnostic tests. Notably, the in-vitro diagnostics (IVD) segment represented approximately 18% of the overall healthcare market in 2023, highlighting its pivotal role in health services.
Therapeutic Instruments: Designed to address medical issues, these tools incorporate various technologies, such as infusion pumps and surgical lasers. The cardiology equipment market, a segment of therapeutic instruments, is projected to exceed $95 billion by 2028, underscoring the growing demand for efficient treatment options.
Monitoring Devices: This category includes heart rate monitors and wearable fitness trackers, which are vital for continuous health management. The global market for monitoring medical instruments was valued at $42.3 billion in 2022 and is expected to experience significant growth, indicating an increasing reliance on these technologies.
Understanding these classifications of types of biomedical devices is essential for healthcare professionals, as it allows them to select the most appropriate tools for their patients. For instance, diagnostic tools like continuous glucose monitors facilitate real-time tracking of glucose levels, enhancing diabetes management and individual outcomes. Similarly, therapeutic instruments such as implantable pacemakers are critical for managing heart conditions, significantly improving individuals' quality of life. As the medical technology landscape evolves, the integration of these tools into clinical practice will continue to transform patient care.

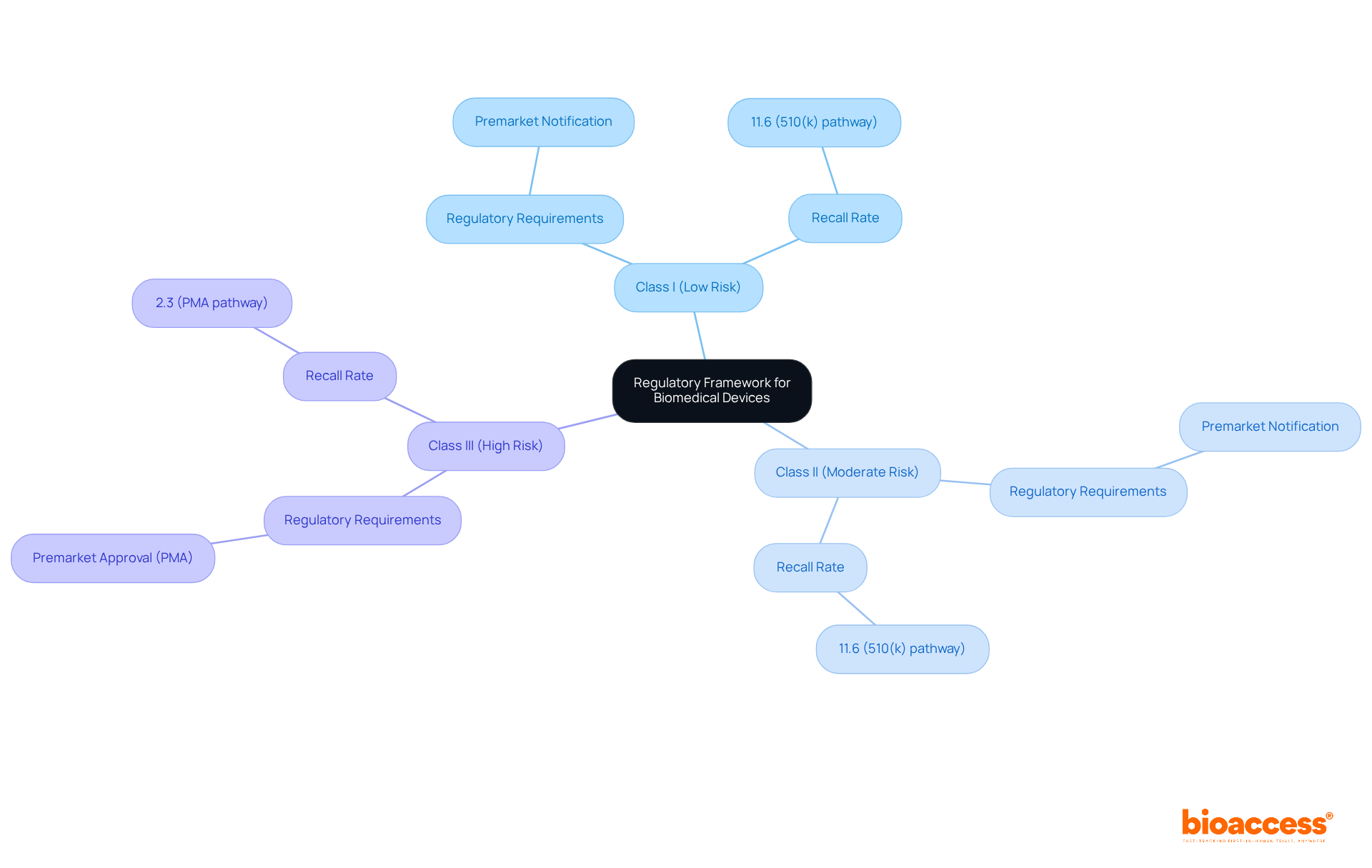
The advancement of biomedical instruments is governed by a stringent framework aimed at ensuring safety and effectiveness. In the United States, the Food and Drug Administration (FDA) categorizes products into three risk-based classes:
Each class entails specific regulatory requirements; for instance, Class I and Class II products require premarket notification, whereas Class III products demand a more rigorous premarket approval (PMA) process. Adhering to these regulations is vital for manufacturers, as it not only facilitates market entry but also cultivates public trust in medical technologies.
Furthermore, the FDA's oversight extends to post-market monitoring, which is essential for evaluating performance and addressing any emerging safety concerns. Notably, the recall rate for PMA-approved products stands at 2.3%, compared to 11.6% for items authorized through the 510(k) pathway, underscoring the varying risk levels associated with different approval processes.
As industry experts Katherine Ruiz and Ana Criado emphasize, compliance with these regulatory standards is crucial for maintaining the integrity of the biomedical equipment sector and safeguarding patient safety.
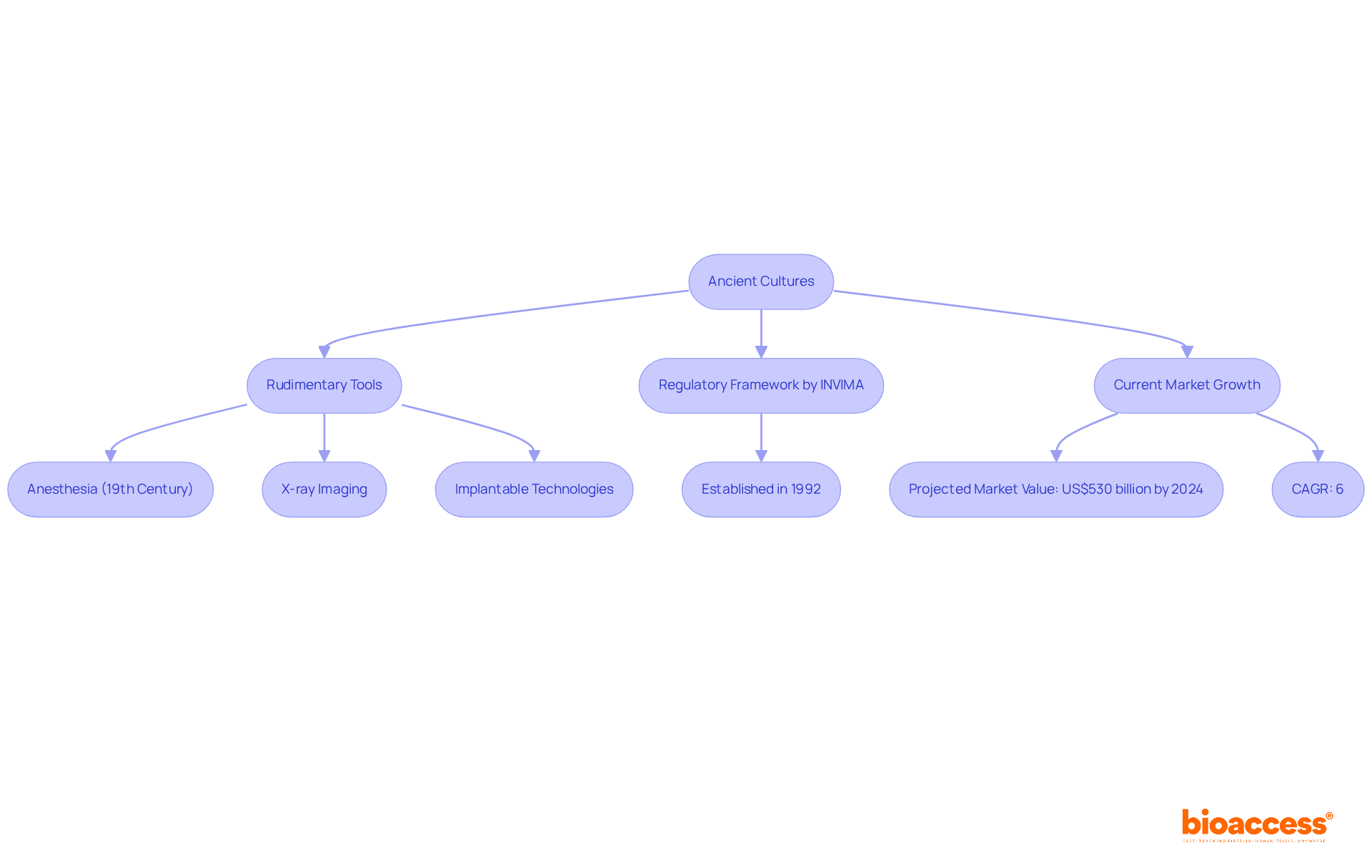
The evolution of types of biomedical devices can be traced back to ancient cultures, where rudimentary tools were employed for health-related purposes. Over the centuries, significant advancements in types of biomedical devices have reshaped the landscape of medical technology. The introduction of anesthesia in the 19th century, the development of X-ray imaging, and the innovation of implantable technologies like pacemakers are all significant advancements that represent types of biomedical devices that have revolutionized patient care. Today, innovations such as telemedicine, wearable health monitors, and AI-driven diagnostic tools represent different types of biomedical devices that continuously push the boundaries of what biomedical technology can achieve.
In Colombia, the regulatory framework governing healthcare products is overseen by INVIMA (Instituto Nacional de Vigilancia de Medicamentos y Alimentos), which plays a vital role in ensuring the safety, efficacy, and quality of these items. Established in 1992 under the Ministry of Health and Social Protection, INVIMA is tasked with inspecting and supervising the marketing and manufacturing of health products. Its Directorate for Healthcare Equipment and other Technologies monitors types of biomedical devices, oversees pre- and post-market programs, and recommends technical standards for their production and quality assurance. Recognized as a Level 4 health authority by the Pan American Health Organization/World Health Organization, INVIMA's rigorous oversight fosters a reliable environment for the growth of various types of biomedical devices in the healthcare equipment market.
In recent years, the market for medical equipment, which encompasses different types of biomedical devices, is projected to reach a value of approximately US$530 billion by 2024, reflecting a compound annual growth rate (CAGR) of around 6%. This growth is driven by the increasing acceptance of digital health innovations and AI-enabled tools, which are important types of biomedical devices essential for meeting the evolving needs of healthcare providers and patients alike. Notably, one in three Americans now utilizes a wearable device, highlighting the influence of these technologies on proactive health management.
Understanding this historical context, along with the regulatory framework established by INVIMA, is crucial for recognizing the rapid pace of technological advancements in the field.
Biomedical devices are integral to modern healthcare, providing essential tools for diagnosis, treatment, and patient monitoring. Their wide-ranging applications, from basic instruments to sophisticated technologies, highlight their vital role in enhancing patient care and improving health outcomes. By comprehensively understanding the various types of biomedical devices and their functionalities, healthcare professionals can make informed decisions that optimize treatment pathways and support individual well-being.
This article underscores key categories of biomedical devices, including diagnostic, therapeutic, and monitoring instruments, each serving a distinct purpose in patient care. It also stresses the necessity of a robust regulatory framework that guarantees the safety and effectiveness of these devices, fostering public trust and facilitating market entry for manufacturers. Moreover, the historical evolution of biomedical devices illustrates the remarkable advancements that have transformed the healthcare landscape, paving the way for innovative solutions that address contemporary health challenges.
Given the increasing reliance on biomedical devices, it is crucial for healthcare providers, regulators, and patients to stay informed about their functionalities and classifications. Embracing these technologies not only enhances clinical decision-making but also empowers individuals to take charge of their health. As the field continues to advance, a commitment to understanding and integrating biomedical devices into practice will be essential for propelling healthcare forward and improving patient outcomes.
What are biomedical devices?
Biomedical devices are tools, machines, implants, and software specifically designed for medical applications, including the diagnosis, prevention, monitoring, and treatment of diseases.
Why are biomedical devices important?
Biomedical devices enhance diagnostic precision, which is crucial for delivering efficient care. They provide critical data that informs treatment strategies and streamline workflows in clinical practice.
Can you provide examples of biomedical devices?
Examples of biomedical devices include basic tools like tongue depressors, sophisticated systems such as pacemakers, continuous glucose monitoring systems like the Dexcom G7, and imaging technologies like MRI and CT scans.
What is the current market trend for in-vitro diagnostics?
The in-vitro diagnostics market was valued at $91.2 billion in 2022 and is anticipated to grow to $118 billion by 2030, indicating an increasing reliance on diagnostic tools.
How do advancements in imaging technologies impact healthcare?
Advancements in imaging technologies, such as MRI and CT scans, have improved diagnostic precision, facilitating earlier detection of conditions like cancer, which affects over 1.7 million Americans annually.
What is the revenue trend for diagnostic imaging equipment?
The sector of diagnostic imaging equipment saw a 6.2% increase in revenue, highlighting its vital role in modern healthcare.
How do consumer health technologies relate to biomedical devices?
Consumer health technologies, such as apps, wearables, and self-diagnosis tools, have the potential to strengthen the patient-physician connection and improve health outcomes, according to healthcare experts.