


Umab drugs, or monoclonal antibodies, represent a significant advancement in medical science, engineered specifically to target distinct antigens within the body. This targeting enhances treatment efficacy for a variety of conditions, including cancer and autoimmune diseases, while simultaneously minimizing adverse effects.
The evolution of umab drugs since the 1970s is marked by pivotal milestones and advancements that have cemented their role as essential components of contemporary therapeutic strategies.
As the market for these drugs continues to expand, their importance in the realm of precision medicine becomes increasingly evident, underscoring the need for ongoing research and collaboration in clinical settings.
Umab drugs, or monoclonal antibodies, signify a groundbreaking advancement in medical science, engineered to precisely target unique antigens within the body. Their human-derived nature enhances compatibility with the immune system and minimizes adverse reactions, making them vital in treating a range of conditions from cancer to autoimmune diseases. As the demand for these targeted therapies continues to rise, particularly in light of increasing cancer rates, it is essential to consider:
Umab drugs, or monoclonal proteins (mAbs), are advanced laboratory-engineered molecules designed specifically to target unique antigens within the body. The 'u' in umab indicates their human-derived nature, significantly enhancing compatibility with the human immune system and minimizing the risk of adverse immune reactions typically associated with non-human proteins. These umab drugs are essential in treating various illnesses, including cancers, autoimmune conditions, and infectious diseases, by binding to specific proteins and modulating the immune response. This targeted approach is fundamental to contemporary medical therapies, enabling more effective and personalized treatment options.
Recent studies highlight the effectiveness of human-derived antibodies, particularly in oncology, where they play a critical role in targeting cancer cells while safeguarding healthy tissue. Notably, the oncology sector commands nearly 50% of the monoclonal antibody market share, driven by the increasing approval of umab drugs for cancer treatment. Furthermore, the anticipated rise in cancer incidence—projected to increase by 47% from 2020 to 2040, reaching approximately 28.4 million new cases globally—underscores the escalating demand for these therapies.
In addition to their applications in cancer treatment, umab drugs are making significant strides in addressing autoimmune disorders. The humanized mAb segment is expected to demonstrate a higher compound annual growth rate (CAGR) due to its reduced immunogenicity compared to chimeric mAbs, rendering them more suitable for long-term treatment regimens. This trend reflects a broader movement toward precision medicine, where therapies are customized to meet individual patient needs, thereby enhancing treatment outcomes and patient satisfaction.
Overall, umab drugs represent a crucial advancement in therapeutic strategies, merging specificity with minimized side effects, thus transforming the treatment landscape for chronic illnesses and improving patient quality of life. The market for targeted therapies is projected to reach approximately USD 729.43 billion by 2033, expanding at a CAGR of 12% from 2025 to 2035, emphasizing the vital role these medications will continue to play in modern healthcare.

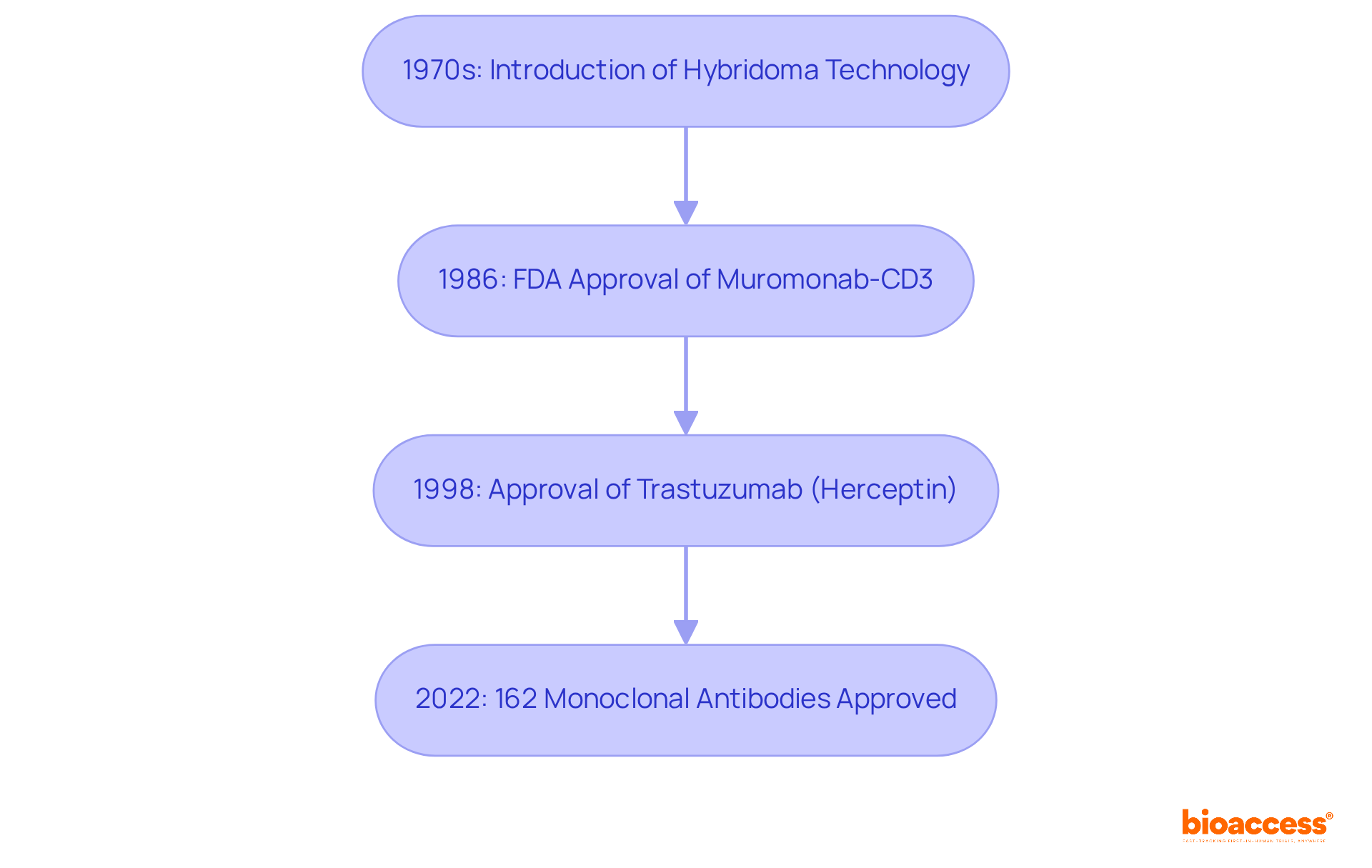
The development of umab medications can be traced back to the 1970s, marked by the groundbreaking launch of hybridoma technology. This innovation enabled the large-scale creation of specific immune proteins, leading to the first therapeutic muromonab-CD3, which received FDA approval in 1986. This pivotal moment in medical history provided a crucial solution for organ transplant rejection. Since then, the landscape of therapeutic proteins has undergone significant transformation, driven by advancements in genetic modification and humanization techniques that have enabled the creation of entirely human-derived proteins. Such evolution has markedly enhanced the efficacy and safety profiles of these therapies.
A landmark achievement occurred in 1998 with the approval of trastuzumab (Herceptin), recognized as the first targeted therapeutic agent specifically authorized for cancer treatment. This milestone not only revolutionized cancer treatment but also ushered in a new era of targeted therapies, demonstrating the potential of specific proteins in addressing complex illnesses.
As of 2022, a total of 162 monoclonal antibodies have received regulatory approval, highlighting their extensive acceptance and therapeutic impact. The umab drugs have evolved into a multi-billion dollar industry, providing a diverse range of products for various therapeutic applications. This evolution reflects their critical role in modern medicine and the continuous innovation within the field.
Umab drugs are characterized by their remarkable specificity and affinity, which are essential for ensuring their therapeutic efficacy. These engineered antibodies are meticulously designed to bind to specific antigens, such as proteins found on the surfaces of cancer cells or inflammatory markers associated with autoimmune disorders. Their high affinity facilitates the effective neutralization or blockade of disease processes, establishing them as powerful tools in modern medicine.
Recent studies underscore the importance of enhancing both affinity and specificity in monoclonal proteins. Research indicates that immune proteins with elevated affinity frequently exhibit increased non-specific binding, which can undermine their therapeutic potential. Thus, achieving a balance between these characteristics is vital for the development of effective monoclonal antibodies. As Peter M. Tessier articulates, "Antibody specificity is critical to the success of antibody therapeutics because non-specific interactions can lead to off-target binding and result in fast antibody clearance."
Engineered monoclonal antibodies can also be optimized to enhance their pharmacokinetic properties, improving their half-life in circulation and tissue penetration while minimizing immunogenicity. Certain monoclonal antibody therapies are conjugated with cytotoxic agents or radioisotopes, allowing for the targeted delivery of therapeutic agents directly to affected cells. This targeted approach not only maximizes therapeutic efficacy but also reduces collateral damage to healthy tissues, significantly enhancing patient outcomes.
Real-world examples of engineered umab drugs demonstrate their transformative impact on treatment protocols. For instance, the advancement of bispecific agents has enabled the concurrent targeting of multiple antigens, thereby improving the efficacy of cancer treatments. Notably, twelve antibodies targeting PD-1 have received approval, demonstrating the effectiveness of this strategy in treating various cancers. Such innovations underscore the ongoing development of umab drugs and their critical role in advancing therapeutic approaches across diverse medical fields.

Umab drugs have emerged as a cornerstone of modern medicine, especially in the fields of oncology, immunology, and infectious diseases. These monoclonal antibodies have revolutionized cancer treatment by offering targeted therapies that significantly enhance survival rates while minimizing side effects compared to traditional chemotherapy.
For instance, adalimumab (Humira), a notable monoclonal antibody, has transformed the treatment of autoimmune conditions, leading to substantial improvements in patient quality of life by effectively alleviating symptoms and preventing disease progression.
Statistics reveal that patients treated with monoclonal antibodies frequently experience a marked decrease in disease activity, with many reporting enhanced daily functioning and overall well-being.
During the COVID-19 pandemic, biological agents played a pivotal role in developing therapies aimed at neutralizing the virus, highlighting their versatility in addressing urgent health challenges.
As research continues to advance, the expanding therapeutic potential of umab drugs solidifies their role in the future of precision medicine.

Umab drugs, or monoclonal antibodies, signify a remarkable leap in targeted therapies, providing tailored treatment options that enhance patient outcomes while minimizing side effects. By honing in on specific antigens, these human-derived proteins have revolutionized modern medicine, particularly in oncology and autoimmune disorders, where their precision and efficacy have proven invaluable.
The evolution of umab drugs is notable, tracing their origins from the 1970s hybridoma technology to their current status as a multi-billion dollar industry. Key milestones, such as the approval of trastuzumab and the rapid expansion in the oncology sector, underscore the increasing reliance on these therapies. Furthermore, the characteristics that define umab drugs—such as specificity, affinity, and the ability to target multiple antigens—are crucial for optimizing treatment protocols and enhancing patient quality of life.
Given the rising prevalence of diseases like cancer and autoimmune disorders, the role of umab drugs in modern healthcare is paramount. As the market for targeted therapies continues to grow, the potential for these innovative treatments to tackle complex medical challenges becomes increasingly significant. Embracing advancements in umab drug development will not only enhance therapeutic strategies but also pave the way for a future where personalized medicine becomes the norm, ultimately improving health outcomes on a global scale.
What are umab drugs?
Umab drugs, or monoclonal proteins (mAbs), are laboratory-engineered molecules designed to target specific antigens in the body. The 'u' in umab indicates that they are human-derived, which enhances compatibility with the human immune system and reduces the risk of adverse immune reactions.
What conditions are umab drugs used to treat?
Umab drugs are essential in treating various illnesses, including cancers, autoimmune conditions, and infectious diseases, by binding to specific proteins and modulating the immune response.
How effective are umab drugs in oncology?
Recent studies show that human-derived antibodies are particularly effective in oncology, as they target cancer cells while protecting healthy tissue. The oncology sector accounts for nearly 50% of the monoclonal antibody market share, driven by the increasing approval of umab drugs for cancer treatment.
What is the projected increase in cancer incidence?
The incidence of cancer is projected to increase by 47% from 2020 to 2040, reaching approximately 28.4 million new cases globally, highlighting the growing demand for umab therapies.
How do umab drugs compare to chimeric mAbs in treating autoimmune disorders?
Humanized mAbs are expected to show a higher compound annual growth rate (CAGR) due to their reduced immunogenicity compared to chimeric mAbs, making them more suitable for long-term treatment regimens.
What is the significance of precision medicine in the context of umab drugs?
The trend toward precision medicine reflects the customization of therapies to meet individual patient needs, which enhances treatment outcomes and patient satisfaction with umab drugs.
What is the projected market value for targeted therapies?
The market for targeted therapies, including umab drugs, is projected to reach approximately USD 729.43 billion by 2033, expanding at a CAGR of 12% from 2025 to 2035.