


Excipients are inactive substances that play a crucial role in drug development by facilitating the manufacturing process, enhancing stability, and improving patient adherence to medications. Their diverse functions, including maintaining stability, improving bioavailability, and enabling controlled release, are essential for effective pharmaceutical formulations. Furthermore, addressing safety concerns and regulatory oversight is vital to ensure their appropriate use in the industry, reinforcing the significance of excipients in optimizing therapeutic outcomes.
Excipients, often overlooked in the realm of pharmaceuticals, represent the unsung heroes that facilitate the effective delivery of active ingredients in medications. These inactive substances comprise an astonishing 90% of drug formulations, enhancing stability and bioavailability while playing a crucial role in patient adherence through taste masking and controlled release.
However, as the complexity of drug development escalates, so too do the challenges associated with these additives, raising significant questions about their safety, potential adverse reactions, and regulatory oversight.
What exactly are excipients, and how do they shape the future of pharmaceuticals?
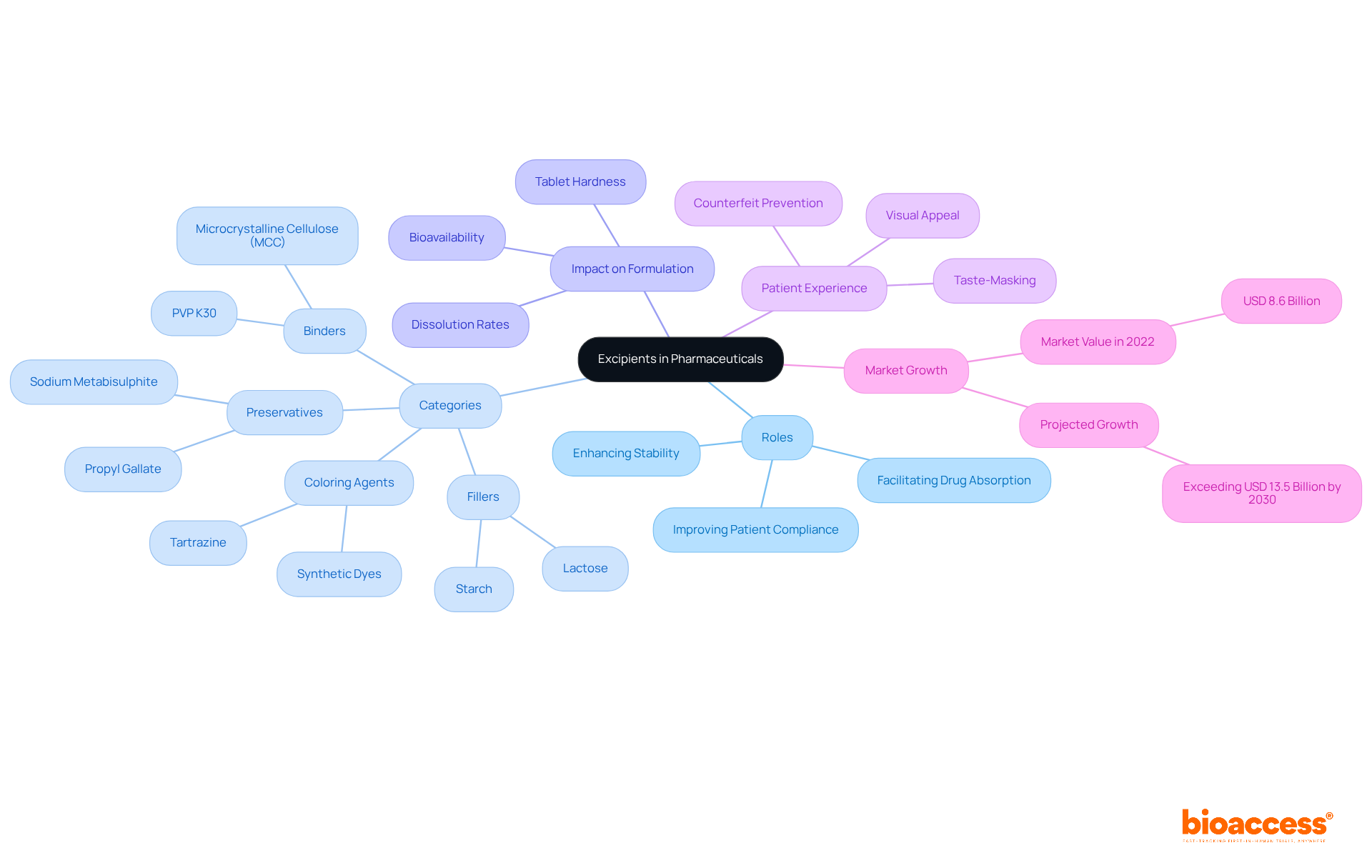
What are excipients? They serve as inactive substances that carry the active components in medications, playing a pivotal role in drug formulation. These substances encompass a diverse range of categories, including binders, fillers, preservatives, and stabilizers. While active pharmaceutical ingredients (APIs) deliver therapeutic effects, the inactive components are essential for facilitating the manufacturing process, enhancing stability, and improving patient adherence. Notably, approximately 90% of medications include inactive ingredients, leading to the inquiry of what are excipients and underscoring their prevalence in medication development.
The influence of additives on medication formulation is substantial, as evidenced by numerous case studies. For example, the selection of a binder can profoundly affect tablet hardness and dissolution rates. Utilizing a robust binder like PVP K30 at high concentrations may lead to a tablet that does not pass dissolution testing, whereas a weaker binder can promote rapid drug release. This scenario illustrates the critical trade-offs in formulation development, where the choice of additives directly impacts both manufacturability and clinical performance.
Moreover, additives significantly enhance patient experience and adherence. Flavoring agents and sweeteners can effectively mask unpleasant tastes, while colorants improve acceptability and assist in preventing counterfeiting. The meticulous selection of additives not only ensures the safety and efficacy of medications but also supports sophisticated delivery systems, thereby enhancing therapeutic outcomes.
Recent findings indicate that the global drug additives market was valued at approximately USD 8.6 billion in 2022, with projections to exceed USD 13.5 billion by the early 2030s. This growth reflects the increasing recognition of additives' roles in improving medication bioavailability and regulating release mechanisms. As the pharmaceutical sector evolves, understanding what are excipients is expected to expand, fostering further innovation in formulation and delivery.
In drug formulation, what are excipients and how do they play a crucial role by serving various essential functions?
Stability is one of their primary contributions; excipients are vital for maintaining the stability of active ingredients, protecting them from degradation caused by environmental factors like moisture and light. For instance, antioxidants are commonly employed to shield sensitive active pharmaceutical ingredients (APIs) from oxidation, thereby prolonging their efficacy.
Bioavailability is another critical aspect, as certain additives significantly enhance the solubility and absorption of drugs, leading to improved bioavailability. Surfactants, for example, can increase the dissolution rate of poorly soluble medications, which is critical for their therapeutic effectiveness. Studies indicate that the use of specific additives can boost bioavailability by up to 50%, underscoring their significance in formulation design.
In pediatric formulations, taste masking is essential; additives are often utilized to conceal unpleasant tastes, making medications more palatable for young patients. This is particularly important in improving adherence to treatment regimens among children, who may be sensitive to bitter flavors.
Controlled release is also a function of some additives, which are designed to enable the gradual release of the active ingredient over time, ensuring sustained therapeutic effects. This capability is essential for managing chronic conditions where consistent medication levels are necessary for efficacy.
Moreover, excipients can enhance manufacturing efficiency by improving the flow properties of powders, streamlining the manufacturing process. Improved flow characteristics lead to more efficient production, reducing the likelihood of batch inconsistencies.
The increasing demand for multifunctionality indicates the necessity for cost efficiency and streamlined manufacturing processes. These additives serve multiple roles in formulations, enhancing stability, bioavailability, and controlled drug release.
Finally, there is a notable shift towards sustainability, with a growing preference for natural, sustainable, and plant-derived substances due to regulatory scrutiny over synthetic additives. This trend highlights the industry's commitment to safer and more environmentally friendly options.
These diverse functions underscore the critical importance of choosing suitable additives, leading to the inquiry of what are excipients to ensure the success and effectiveness of pharmaceutical products.

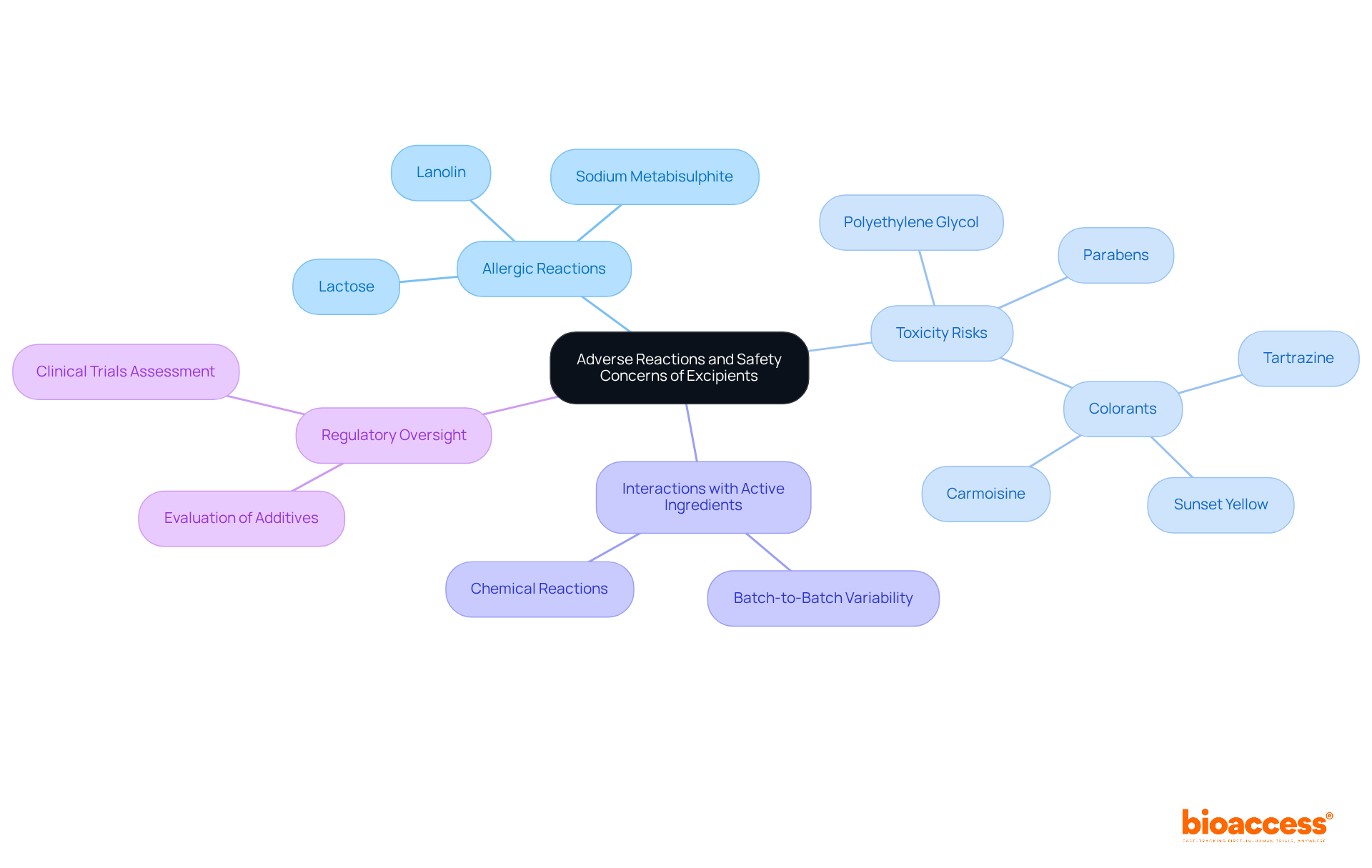
What are excipients that, while generally deemed safe, can occasionally lead to adverse reactions in patients? Key safety concerns warrant attention:
Allergic Reactions: Excipients such as lactose and sodium metabisulphite are known to provoke allergic responses in sensitive individuals, highlighting the necessity for careful patient assessment.
Toxicity Risks: Understanding what are excipients is crucial, as certain excipients, particularly colorants and preservatives, have been associated with toxicity when used inappropriately or in excessive quantities. For instance, prolonged exposure to polyethylene glycol or parabens raises concerns regarding potential toxicity in high doses.
Interactions with Active Ingredients: Excipients can engage with active pharmaceutical ingredients (APIs) or other medications, potentially undermining their efficacy or security. This underscores the importance of compatibility studies during formulation development. Additionally, batch-to-batch variability can alter drug performance due to inconsistent properties of what are excipients.
Regulatory Oversight: Regulatory bodies require thorough evaluation of additives to verify their reliability and compatibility with APIs. This includes assessing their potential to induce adverse effects during clinical trials. Progress in formulation design and improved regulatory frameworks are essential to tackle the challenges presented by these substances.
Comprehending these risk issues is essential for medication developers; addressing them can significantly reduce hazards and enhance patient well-being throughout the treatment development process. The importance of what are excipients, which constitute around 90% of a drug's formulation and represent 0.5% of the overall medicine market, in facilitating efficient drug delivery and ensuring patient safety cannot be overstated.
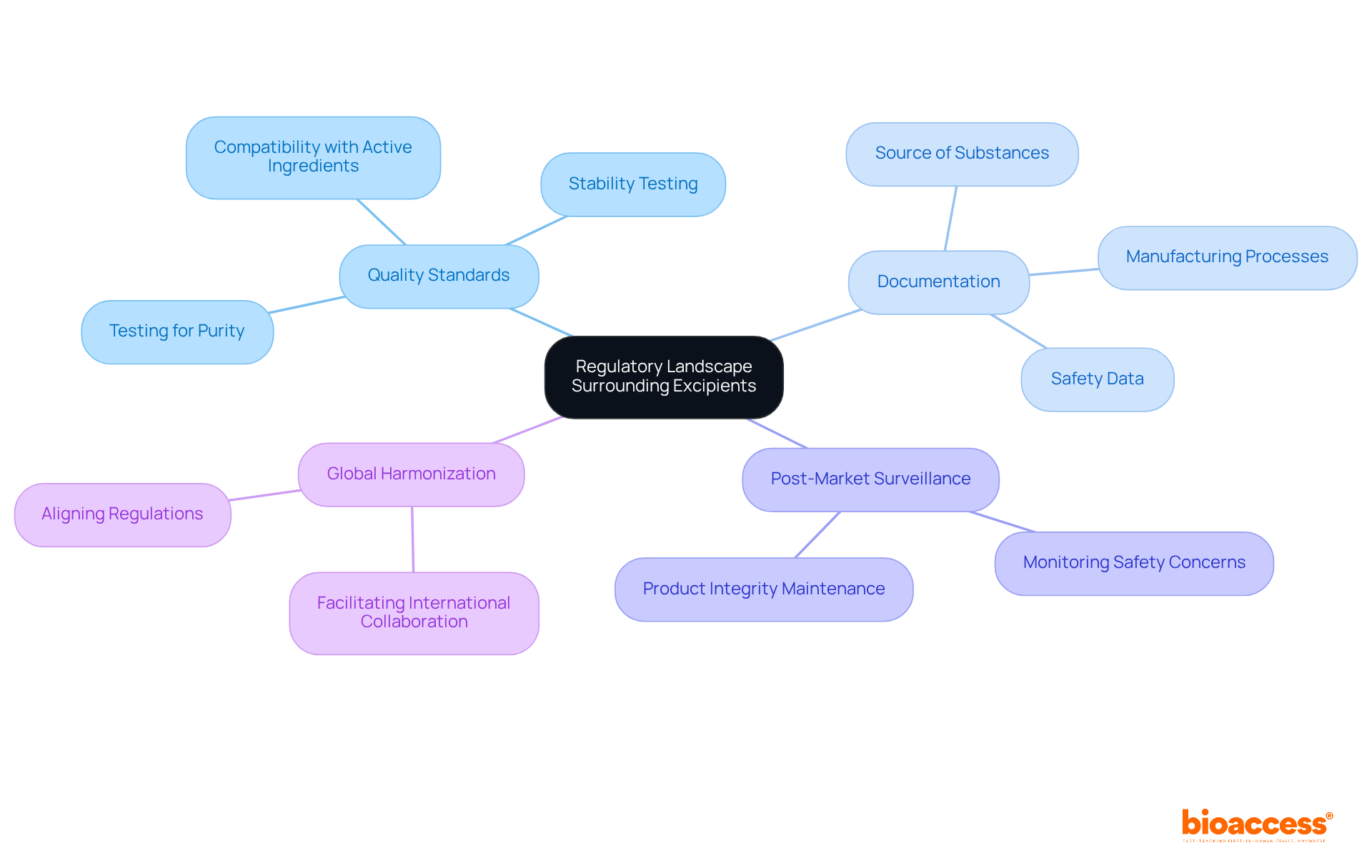
The regulatory environment for additives is complex and varies significantly across regions, including Colombia. Regulatory bodies, such as INVIMA (Colombia National Food and Drug Surveillance Institute), play a crucial role in overseeing the evaluation and approval of what are excipients used in pharmaceuticals. Classified as a Level 4 health authority by PAHO/WHO, INVIMA is responsible for inspecting and overseeing the marketing and production of health products, ensuring adherence to standards of quality and efficacy.
Key considerations include:
Quality Standards: Excipients must adhere to stringent quality standards to guarantee their safety and efficacy. This encompasses rigorous testing for purity, stability, and compatibility with active pharmaceutical ingredients. Adhering to INVIMA's guidelines helps clarify what are excipients and ensures that additives meet the necessary criteria for use in drug formulations in Colombia.
Documentation: Manufacturers are required to provide comprehensive documentation concerning the substances used in their formulations. This includes details about their source, manufacturing processes, and security data, which are critical for regulatory review and approval by authorities like INVIMA.
Post-Market Surveillance: Continuous monitoring of excipients is essential once a drug reaches the market. This ongoing surveillance helps identify any potential safety concerns that may arise, ensuring that the integrity of the product is maintained throughout its lifecycle.
Global Harmonization: Ongoing efforts aim to align regulations across different regions, simplifying the worldwide development and approval processes for drugs. Such harmonization is vital for facilitating international collaboration and ensuring that high-quality standards are upheld globally.
Navigating this regulatory framework, particularly with the expertise of professionals like Ana Criado, Director of Regulatory Affairs and a consultant for global companies, is imperative for pharmaceutical companies to ensure compliance and uphold the integrity of their products. Ana's extensive background in regulatory affairs, including her leadership roles at INVIMA and her academic credentials, further solidifies her authority in this field. This ultimately contributes to the safety and efficacy of medications available to patients.
Excipients play an indispensable role in drug development, serving as the backbone of pharmaceutical formulations. These inactive substances not only facilitate the delivery of active pharmaceutical ingredients (APIs) but also enhance stability, bioavailability, and patient adherence. Understanding the multifaceted functions of excipients is crucial for ensuring the efficacy and safety of medications, as they constitute a significant portion of drug formulations.
Key insights reveal that excipients contribute to various essential aspects of drug formulation, such as:
Their impact on patient experience—through taste masking and enhanced acceptability—further emphasizes the importance of careful selection in the formulation process. Additionally, the growing market for drug additives highlights the increasing recognition of their vital role in pharmaceutical innovation.
In light of these findings, it is paramount for stakeholders in the pharmaceutical industry to prioritize the understanding and strategic use of excipients. As the regulatory landscape evolves, ongoing research and development will be essential to address safety concerns and optimize drug formulations. Embracing advancements in excipient technology will not only enhance drug efficacy but also contribute to improved patient outcomes and a more sustainable pharmaceutical future.
What are excipients in pharmaceuticals?
Excipients are inactive substances that carry the active components in medications and play a crucial role in drug formulation.
What categories do excipients encompass?
Excipients include a diverse range of categories such as binders, fillers, preservatives, and stabilizers.
Why are excipients important in medication?
Excipients facilitate the manufacturing process, enhance stability, and improve patient adherence, making them essential for effective medication development.
How prevalent are excipients in medications?
Approximately 90% of medications include inactive ingredients, highlighting the significant role of excipients in drug formulation.
How do additives influence medication formulation?
Additives can substantially affect properties such as tablet hardness and dissolution rates, impacting both manufacturability and clinical performance.
Can you provide an example of how a binder affects medication?
The choice of binder can influence tablet characteristics; for instance, a robust binder like PVP K30 at high concentrations may result in tablets failing dissolution testing, while a weaker binder may promote faster drug release.
How do additives enhance patient experience?
Flavoring agents and sweeteners can mask unpleasant tastes, while colorants improve acceptability and help prevent counterfeiting.
What is the current market value of drug additives?
The global drug additives market was valued at approximately USD 8.6 billion in 2022, with projections to exceed USD 13.5 billion by the early 2030s.
Why is the understanding of excipients expected to grow in the pharmaceutical sector?
As the pharmaceutical sector evolves, there is increasing recognition of additives' roles in improving medication bioavailability and regulating release mechanisms, which fosters further innovation in formulation and delivery.