


Argentina is rapidly emerging as a pivotal hub for medical technology clinical trials, attracting attention from both domestic and international stakeholders. This transformation is fueled by a robust regulatory environment, a highly skilled workforce, and a cost-effective operational landscape that collectively enhance the country’s appeal for clinical research.
The Argentine government has proactively implemented policies aimed at fostering innovation and research, resulting in heightened foreign investment within the medtech sector. With the expertise of organizations like bioaccess®, which offers comprehensive clinical trial management services, Argentina is well-positioned to meet the growing demands of medical device companies.
The unique demographic diversity of its patient population further enriches the clinical trial landscape, enabling thorough data collection and analysis. As the country solidifies its role in the global medtech arena, it not only contributes to economic growth and job creation but also improves healthcare outcomes, setting the stage for a promising future in medical device research.
In recent years, Argentina has positioned itself as a developing center for medical technology research, particularly in the realm of Medical Device CRO Argentina, attracting attention from both local and global stakeholders. This transformation is significantly influenced by the country's robust regulatory framework, skilled workforce, and cost-effective operational environment. The Argentine government has enacted policies to foster innovation and research, leading to increased foreign investment in the medtech sector.
Moreover, with the expertise of companies like bioaccess®, which focuses on comprehensive study management services—including:
Argentina's capabilities are improved. The diverse patient population also provides a distinctive benefit for studies, enabling thorough data gathering across different demographics. Consequently, numerous healthcare device firms are progressively seeking the assistance of Medical Device CRO Argentina for their research requirements, establishing it as an important participant in the global medtech sector while aiding local job creation, economic development, and enhanced healthcare results.
Furthermore, the partnership between Greenlight Guru and bioaccess® enhances the advancement and implementation of medical studies, strengthening Argentina's position in the medtech sector.
Device Contract Research Organizations (CROs), such as Medical Device CRO Argentina, are vital to the successful implementation of clinical studies, serving as crucial intermediaries between device manufacturers and regulatory bodies. With approximately two million different medical devices currently available in the global market, the role of the Medical Device CRO Argentina becomes increasingly vital in navigating this complex landscape. Organizations like bioaccess® provide a comprehensive suite of services that includes:
Each of these services plays an essential role in ensuring that studies adhere to both local and global standards. For example, experiment preparation entails thorough planning and coordination to ensure all logistics and compliance requirements are satisfied, while adherence assessments are crucial for aligning with ethical standards and legal mandates. By utilizing comprehensive understanding of regulatory requirements, CROs ensure that studies enhance the credibility and reliability of research findings.
Moreover, CROs play an essential role in facilitating communication among key stakeholders such as sponsors, investigators, and ethics committees, ensuring alignment and transparency throughout the research process. The participation of CROs not only simplifies processes but also greatly diminishes the risks linked to research studies, ultimately aiding in the successful development and approval of health devices. In addition to these services, medical device startups face significant challenges in Argentina, including regulatory hurdles, competition from established players, recruitment issues, and limited financial resources, which a Medical Device CRO Argentina can help mitigate.
Addressing these challenges is crucial for startups aiming to succeed in the MedTech sector. In 2024, companies such as Lindus Health are at the forefront by transforming research services with a strong emphasis on patient involvement and digital health technologies. As mentioned by Lindus Health, 'Lindus Health is a CRO that is transforming the domain of research services.'
Their innovative approach enhances patient engagement and study efficiency, resulting in high-quality data collection that is crucial for the advancement of the MedTech sector. Furthermore, bioaccess® has greatly influenced local economies through job creation, economic growth, and healthcare enhancement, positioning itself as a leader in Medtech research in Latin America with an emphasis on innovation and compliance excellence.
The oversight framework overseeing healthcare equipment assessments in Argentina is mainly administered by the National Administration of Drugs, Food and Medical Technology (ANMAT). This governing body plays a crucial role in approving clinical study protocols and ensuring compliance with both national and international standards. A key aspect of this governing framework includes stringent labeling requirements for healthcare devices, which mandate that all labels must include product descriptions, storage information, expiration dates, and importer details, all presented in Spanish.
For the Medical Device CRO Argentina engaged in medical device studies, maneuvering through this complex compliance environment is crucial. Key requirements include:
These components are essential for carrying out experiments that are ethical, transparent, and consistent with compliance expectations.
At bioaccess®, we provide extensive clinical trial management services that include:
This ensures that our clients meet the necessary legal benchmarks effectively. Additionally, we provide review and feedback on study documents to comply with country requirements, further enhancing our service offerings. Katherine Ruiz, our specialist in compliance matters for healthcare devices and in vitro diagnostics, offers invaluable insights to this process, having advised numerous foreign producers on obtaining market clearance in Colombia.
Furthermore, a relevant case study titled 'Labeling Requirements for Medical Devices' illustrates how these labeling mandates promote safety and compliance, ensuring that consumers and healthcare providers have essential information about the medical devices. It is also noteworthy that electronic signatures are tagged five times within the oversight processes, highlighting their significance in maintaining compliance. Given the dynamic nature of regulations, it is imperative for the Medical Device CRO Argentina to stay updated on any amendments that could impact study timelines and strategic planning.
This vigilance not only promotes adherence to regulations but also improves the overall integrity of the research process in the MedTech sector.
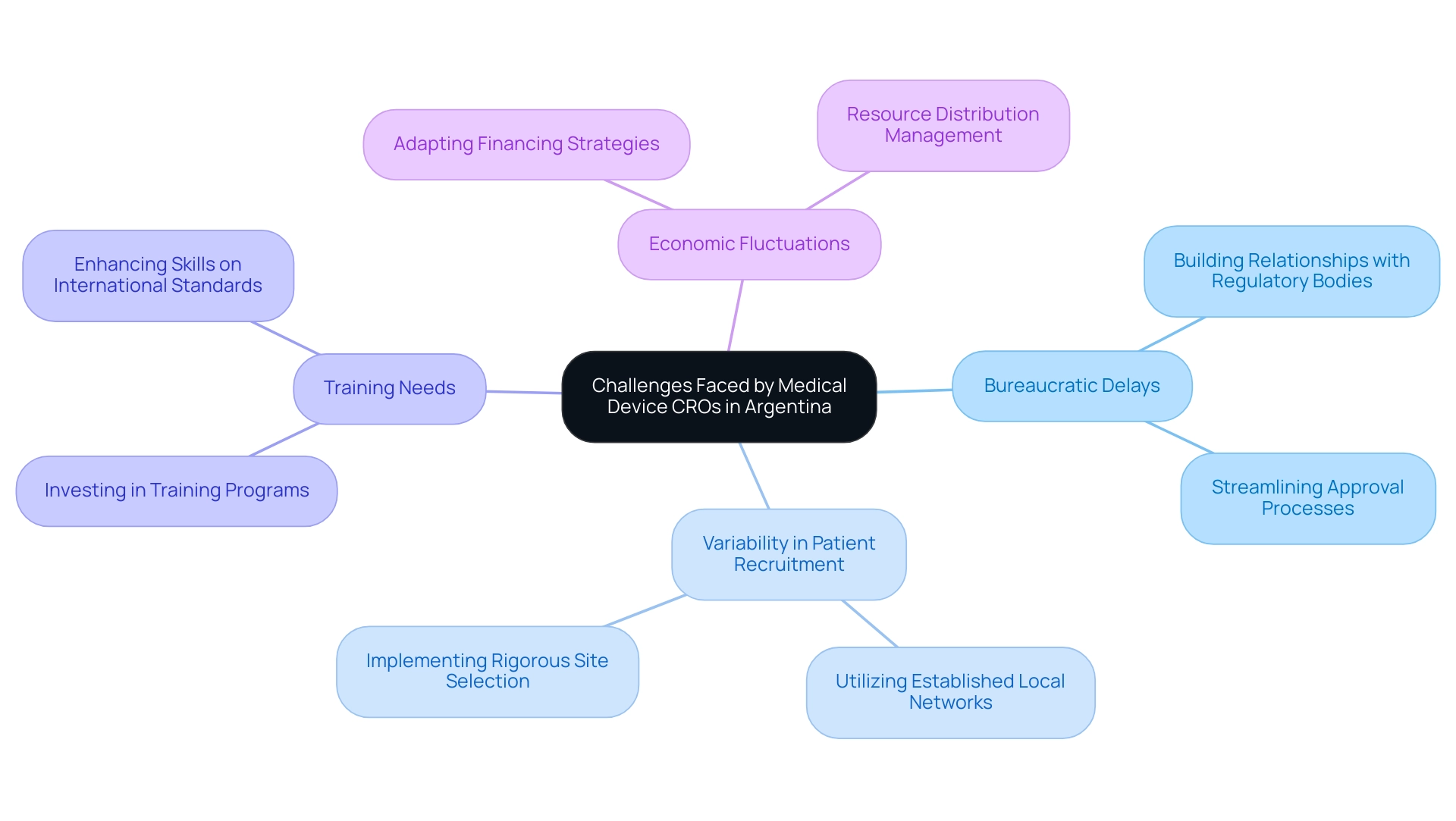
Despite the benefits, the Medical Device CRO Argentina encounters various obstacles that can complicate the research process. These include:
Economic fluctuations in Argentina can also affect financing and resource distribution for research studies.
However, companies like bioaccess® leverage over 20 years of Medtech expertise to effectively navigate the challenges in the Medical Device CRO Argentina. By providing comprehensive clinical study management services—including:
bioaccess® can significantly enhance study efficiency. They implement structured methodologies such as detailed feasibility assessments, rigorous site selection, and compliance reviews to streamline setup and execution.
To mitigate local challenges, they build strong relationships with regulatory bodies and utilize established local networks for patient recruitment, ensuring a robust and efficient recruitment process. Additionally, bioaccess® invests in training programs to enhance the skills of their teams. By proactively tackling these obstacles, bioaccess® not only enhances the success rates of research studies but also promotes job creation, economic growth, and healthcare advancements in the regions they operate.
The future of medical device evaluations in Argentina is set for substantial change, especially with the involvement of Medical Device CRO Argentina, primarily driven by technological advancements. The integration of artificial intelligence into data analysis and patient recruitment processes is set to enhance the efficiency and effectiveness of Contract Research Organizations (CROs) like bioaccess®. With more than 20 years of expertise, bioaccess® focuses on thorough study management services, including:
Additionally, bioaccess® offers:
As highlighted in Michael Baumann's review, optimizing research methodologies could result in substantial financial benefits for biopharmaceutical companies, evidenced by potential double-digit improvements in net present value. Moreover, Argentina's dedication to enhancing its regulatory system and promoting international partnerships is anticipated to result in a rise in research studies. The growing focus on patient-centered research will encourage CROs to adopt innovative methodologies, thereby enhancing patient engagement and outcomes.
The inclusion of real-world data and AI-driven strategies offers a more objective method for choosing indications for specific molecules, significantly enhancing the trial landscape. Case studies such as 'Causal Machine Learning in Evidence Generation' exemplify the power of combining biostatistics with machine learning to distinguish correlation from causation in medical research. These emerging trends position Medical Device CRO Argentina as essential contributors to the global MedTech ecosystem, underscoring their vital role in advancing the future of clinical research.
Argentina's emergence as a pivotal hub for medical technology clinical trials is underscored by a combination of favorable regulatory conditions, a skilled workforce, and a diverse patient demographic. The proactive policies implemented by the Argentine government have not only attracted significant foreign investment but have also positioned organizations like bioaccess® as leaders in clinical trial management. Their comprehensive services, from feasibility studies to compliance reviews, enhance the efficiency and credibility of clinical research, ensuring that medical device companies can navigate the complexities of the regulatory landscape with confidence.
Despite the challenges that medical device Contract Research Organizations (CROs) face, including bureaucratic delays and economic fluctuations, the strategic efforts of companies like bioaccess® to build robust networks and invest in staff training demonstrate resilience and adaptability. These initiatives not only improve trial success rates but also foster local economic growth and better healthcare outcomes.
Looking ahead, the future of medical device clinical trials in Argentina appears bright. The integration of advanced technologies, such as artificial intelligence, is set to revolutionize data analysis and patient recruitment, further enhancing the capabilities of CROs. As the country continues to strengthen its regulatory framework and embrace innovative methodologies, it is well-positioned to play an increasingly vital role in the global MedTech landscape. The ongoing commitment to patient-centered research and the incorporation of real-world data will undoubtedly lead to improved clinical trial outcomes, solidifying Argentina's status as a key player in the advancement of medical technology research.
What is the current state of medical technology research in Argentina?
Argentina has become a developing center for medical technology research, particularly in the area of Medical Device CROs, gaining attention from both local and global stakeholders due to its robust regulatory framework, skilled workforce, and cost-effective operational environment.
How is the Argentine government supporting the medtech sector?
The Argentine government has enacted policies to foster innovation and research, which has led to increased foreign investment in the medical technology sector.
What services does bioaccess® provide in the context of medical device studies?
Bioaccess® offers comprehensive study management services, including early-feasibility studies, first-in-human studies, feasibility assessments, site selection, and compliance reviews.
What advantages does Argentina's patient population offer for medical studies?
The diverse patient population in Argentina allows for thorough data gathering across different demographics, which is beneficial for medical studies.
What role do Medical Device CROs play in clinical studies?
Medical Device CROs serve as intermediaries between device manufacturers and regulatory bodies, facilitating the implementation of clinical studies and ensuring compliance with local and global standards.
What are some of the key services provided by Medical Device CRO Argentina?
Key services include feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting.
What challenges do medical device startups face in Argentina?
Startups in Argentina encounter regulatory hurdles, competition from established players, recruitment issues, and limited financial resources.
Who oversees healthcare equipment assessments in Argentina?
The National Administration of Drugs, Food and Medical Technology (ANMAT) oversees healthcare equipment assessments, approving clinical study protocols and ensuring compliance with standards.
What are the key requirements for conducting medical device studies in Argentina?
Key requirements include securing ethical committee approvals, obtaining informed consent from participants, and complying with Good Clinical Practice (GCP) guidelines.
How does bioaccess® address the challenges faced by medical device CROs in Argentina?
Bioaccess® navigates challenges by providing extensive clinical trial management services, building strong relationships with regulatory bodies, utilizing local networks for patient recruitment, and investing in training programs for local staff.
What future advancements are expected in medical device evaluations in Argentina?
The future of medical device evaluations is expected to be driven by technological advancements, including the integration of artificial intelligence in data analysis and patient recruitment, enhancing the efficiency of CROs.
How does Argentina's regulatory system impact research studies?
Argentina's dedication to improving its regulatory system and promoting international partnerships is anticipated to lead to an increase in research studies and enhance patient-centered research methodologies.