

Medical devices encompass a broad spectrum of healthcare instruments designed for diagnosing, preventing, monitoring, treating, or alleviating health conditions. This range includes everything from basic tools like bandages to advanced technologies such as MRI machines. Understanding these devices is crucial for innovators, as they play a pivotal role in enhancing patient care.
The medical device market is poised for significant growth, projected to reach approximately $678.88 billion by 2025. This growth is driven by technological advancements and an increasing demand for innovative healthcare solutions, underscoring the importance of staying informed in this dynamic field.
Understanding the landscape of medical devices is crucial in a world increasingly reliant on technology for health solutions. These instruments, which range from simple bandages to advanced robotic surgical systems, play a pivotal role in diagnosing, treating, and managing health conditions.
As the industry rapidly evolves, innovators face the challenge of navigating complex regulatory frameworks and staying ahead of technological advancements.
What does it truly mean to innovate within this space? How can one ensure that their contributions enhance patient outcomes while adhering to safety standards?
This exploration not only highlights the significance of innovation but also underscores the necessity for collaboration in overcoming the challenges faced in clinical research.
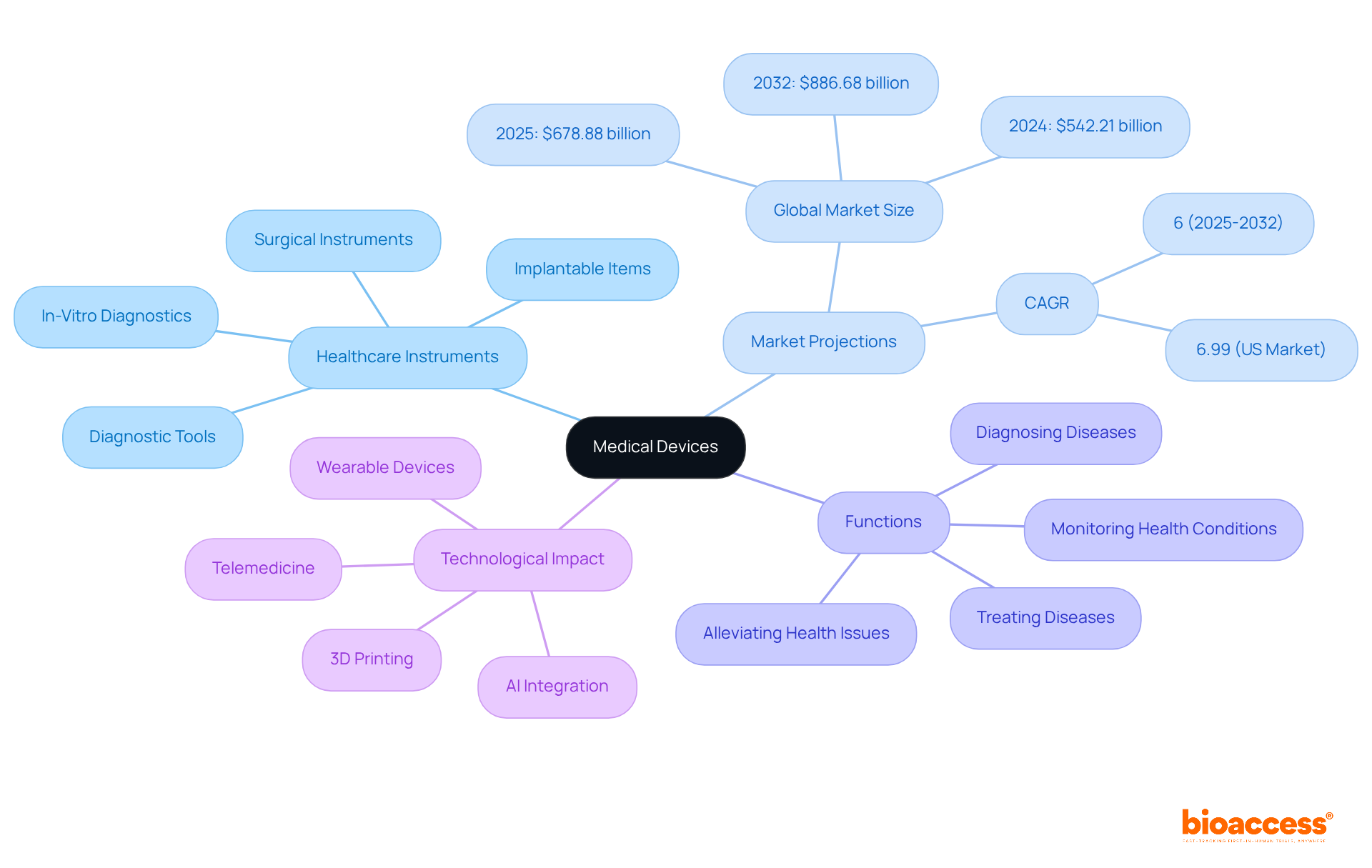
Healthcare instruments are examples of what are medical devices, encompassing a diverse array of tools, apparatuses, machines, appliances, implants, reagents for in vitro use, software, and materials specifically designed for health-related applications. This category spans from fundamental items like bandages and thermometers to sophisticated technologies such as MRI machines and robotic surgical systems. Their primary functions include diagnosing, preventing, monitoring, treating, or alleviating diseases and health conditions, which leads to the inquiry of what are medical devices and their critical role in healthcare delivery and patient outcomes. For innovators, understanding what are medical devices is vital, as it delineates the spectrum of healthcare instruments and underscores their significant potential to enhance patient care.
The healthcare equipment sector is projected to reach approximately $678.88 billion by 2025, growing at a compound annual growth rate (CAGR) of 6%. This expansion is propelled by technological advancements and an escalating demand for innovative healthcare solutions. For example, AI-powered surgical robots are transforming surgical precision and reducing recovery times, illustrating how technology can directly enhance patient outcomes.
In terms of classification, what are medical devices can be categorized into various groups, including healthcare tools, diagnostic tools, surgical instruments, and implantable items. Notably, the diagnostic imaging sector alone accounts for about 20% of the total healthcare equipment market, emphasizing its crucial role in modern healthcare.
Expert opinions underscore the transformative impact of instruments on healthcare provision. As the industry evolves, the integration of digital health technologies, such as telemedicine and wearable devices, is expected to further enhance patient monitoring and treatment efficiency. Understanding the intricate characteristics of healthcare instruments is essential for creators aiming to navigate this dynamic landscape and improve health outcomes.
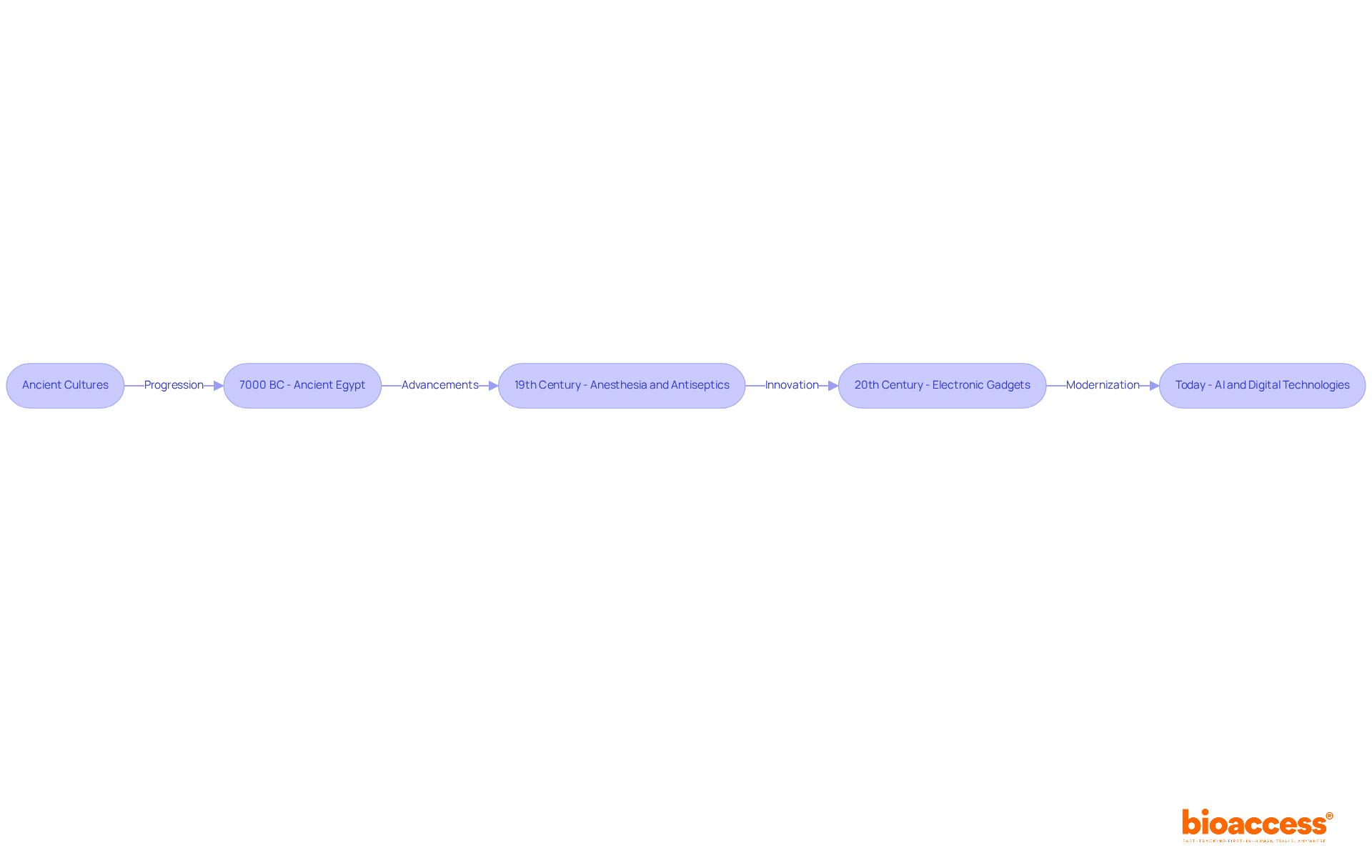
The development of healthcare instruments can be traced back to ancient cultures, where early humans employed basic tools for healing and surgery. Archaeological evidence from ancient Egypt reveals the use of various medical instruments as early as 7000 BC. The 19th century marked significant advancements with the introduction of anesthesia and antiseptics, leading to safer surgical procedures. Notably, FDA endorsements for products such as Fresenius Medical Care's 5008X Hemodialysis System and Boston Scientific's WaveWriter spinal cord stimulator systems illustrate the continuous advancement in this area. The 20th century witnessed the emergence of electronic gadgets, such as pacemakers and imaging technologies, transforming diagnostics and treatment. Today, the combination of artificial intelligence and digital technologies propels innovation, making healthcare instruments more efficient and accessible.
In Colombia, the regulatory environment is influenced by INVIMA (Colombia National Food and Drug Surveillance Institute), which supervises the marketing and production of wellness products, ensuring adherence to safety standards. As a Level 4 health authority acknowledged by the Pan American Health Organization/World Health Organization, INVIMA plays an essential role in overseeing healthcare products and proposing technical standards for quality assurance.
The Internet of Medical Things (IoMT) market, projected to grow from $30.79 billion in 2021 to $187.60 billion by 2028, reflects a shift towards more connected and data-driven healthcare solutions. Understanding what are medical devices in this historical context is crucial for innovators, as it emphasizes the significance of ongoing enhancement and flexibility in the healthcare equipment sector.
As historian Dr. Jane Smith notes, 'The development of medical instruments has always been a reflection of society's evolving understanding of health and disease.' This perspective underscores the necessity for continuous advancements to meet the changing needs of healthcare.
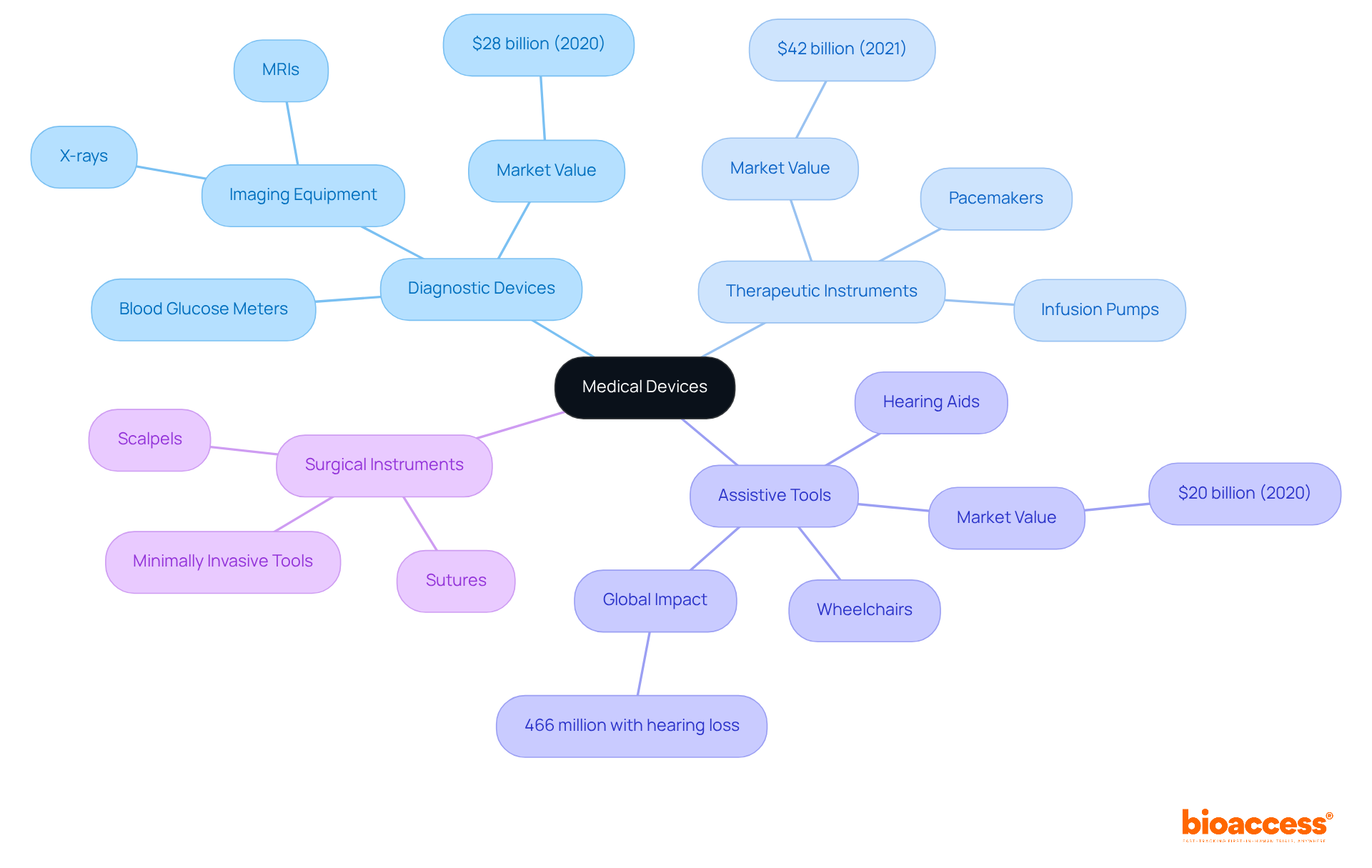
To understand what are medical devices, it is important to note that medical instruments are categorized into various groups according to their intended application and intricacy, each contributing significantly to healthcare innovation. The primary categories include:
Diagnostic Devices: Essential for detecting and diagnosing diseases, these instruments include blood glucose meters, vital for diabetes management, and imaging equipment like X-rays and MRIs, which facilitate accurate disease identification. The diagnostic imaging sector alone was valued at approximately $28 billion in 2020, reflecting the significant demand for these technologies.
Therapeutic Instruments: Designed to treat or manage medical conditions, therapeutic instruments encompass a range of technologies, including pacemakers that regulate heart rhythms and infusion pumps that deliver medications. The therapeutic equipment market was valued at approximately $42 billion in 2021, highlighting their significance in patient care.
Assistive Tools: Supporting individuals with disabilities, these tools enhance quality of life. Common examples include wheelchairs and hearing aids, crucial for mobility and communication. With over 466 million people globally suffering from disabling hearing loss, hearing aids significantly improve communication abilities and overall quality of life.
Surgical Instruments: This category includes tools used during surgical procedures, such as scalpels and sutures. The advent of minimally invasive surgical tools has transformed surgical practices, leading to reduced recovery times and lower complication risks.
In Colombia, the regulation of these instruments falls under the authority of INVIMA (Instituto Nacional de Vigilancia de Medicamentos y Alimentos), which supervises the promotion and production of wellness products. As a Level 4 health authority acknowledged by the Pan American Health Organization/World Health Organization, INVIMA guarantees that healthcare products adhere to safety, efficacy, and quality standards. Understanding what are medical devices is essential for innovators, as it guides product development, regulatory pathways, and positioning within the industry. The healthcare equipment sector is anticipated to surpass $700 billion by 2030, with diagnostic tools accounting for a substantial portion owing to their essential function in early illness identification and management. As the industry evolves, staying abreast of innovations and market trends will be essential for success.
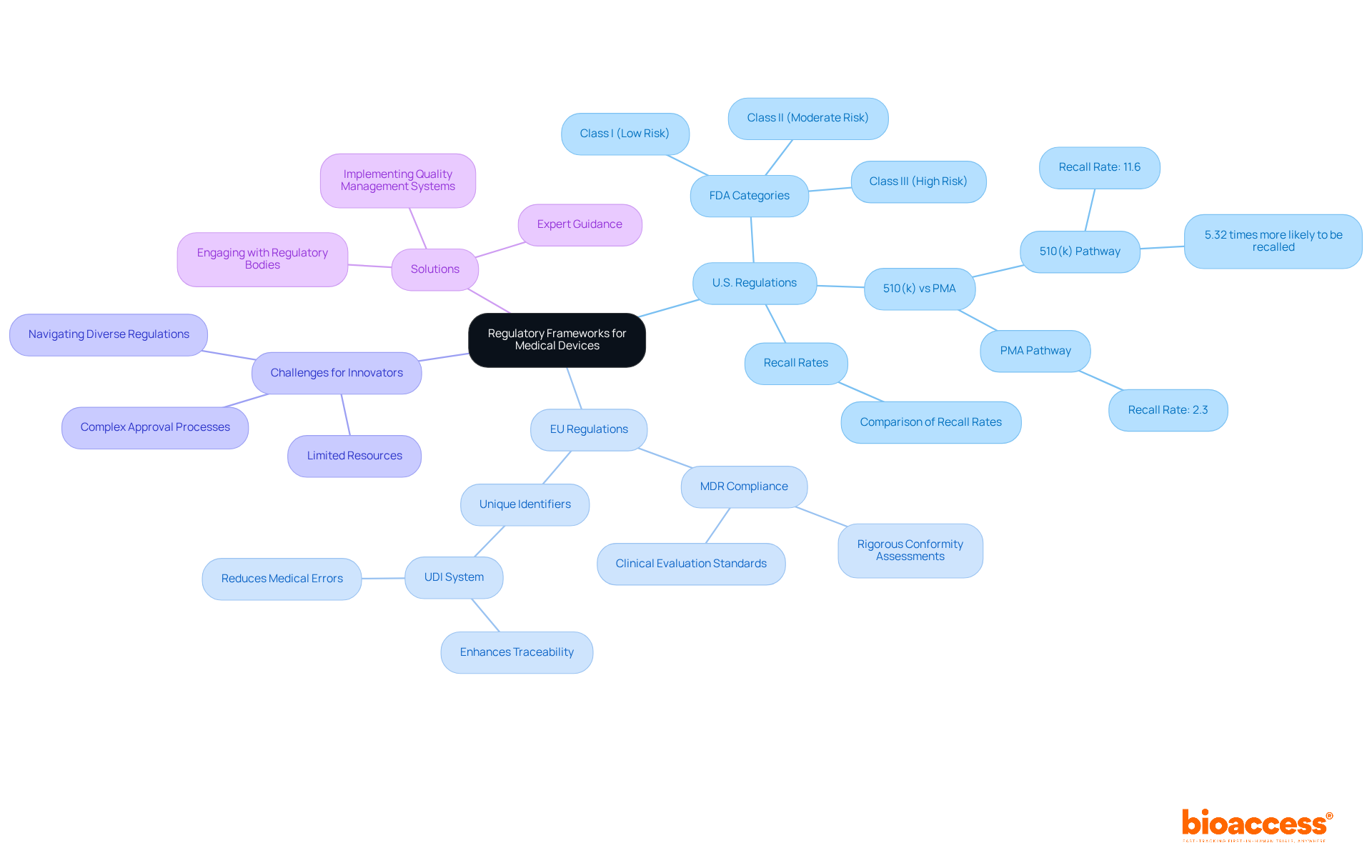
The regulatory environment for healthcare instruments is intricate and varies significantly across regions. In the United States, the Food and Drug Administration (FDA) classifies medical instruments into three categories based on risk:
Each category entails specific regulatory requirements, including premarket notification (510(k)) or premarket approval (PMA) processes. Notably, products authorized through the 510(k) pathway are 5.32 times more likely to be recalled than those approved via PMA, underscoring the critical nature of these classifications in ensuring product safety.
In the European Union, the Medical Devices Regulation (MDR) enforces rigorous conformity assessments to ensure safety and performance. Compliance with the MDR is crucial, as it not only protects patient health but also enhances market access and builds trust among stakeholders. The MDR has introduced improved clinical evaluation standards and mandates that healthcare instruments have unique identifiers for better traceability, further strengthening the regulatory framework.
Grasping these regulatory frameworks is essential for innovators striving to understand what are medical devices and navigate the landscape effectively. Smaller companies, in particular, may encounter significant challenges in maneuvering through diverse regulatory environments due to limited resources, competition from established firms, and difficulties in patient recruitment. Engaging with regulatory bodies early and seeking expert guidance, such as that offered by bioaccess®, can greatly streamline the approval process, reducing potential delays and ensuring compliance with safety standards. Bioaccess® provides customized solutions, including regulatory consulting and patient recruitment strategies, to assist startups in overcoming these obstacles. Furthermore, implementing a robust document and quality management system (QMS) is vital for maintaining data integrity and managing documentation efficiently. As the landscape continues to evolve, remaining informed about regulatory changes and compliance rates is essential for successful market entry and sustained innovation.
Understanding the multifaceted nature of medical devices is essential for innovators aiming to navigate the healthcare landscape effectively. These instruments, ranging from basic tools to advanced technologies, play a pivotal role in diagnosing, treating, and managing health conditions. Their significance cannot be overstated, as they directly impact patient outcomes and the overall quality of care.
This article delves into the historical evolution of medical devices, highlighting key advancements and the regulatory frameworks that govern their use. From ancient healing tools to modern AI-powered surgical robots, the progression of these technologies illustrates the ongoing commitment to improving healthcare. Moreover, the categorization of medical devices into diagnostic, therapeutic, assistive, and surgical tools underscores their diverse applications and importance in enhancing patient care.
As the medical device industry continues to evolve, staying informed about current trends and regulatory requirements becomes increasingly vital. Innovators are encouraged to engage with regulatory bodies and leverage expert guidance to ensure compliance and foster innovation. By understanding the landscape of medical devices, stakeholders can contribute to the advancement of healthcare solutions that not only meet regulatory standards but also significantly improve patient health outcomes.
What are medical devices?
Medical devices are healthcare instruments designed for health-related applications, including tools, apparatuses, machines, appliances, implants, reagents for in vitro use, software, and materials. They range from simple items like bandages and thermometers to complex technologies like MRI machines and robotic surgical systems.
What functions do medical devices serve?
The primary functions of medical devices include diagnosing, preventing, monitoring, treating, or alleviating diseases and health conditions.
Why is it important for innovators to understand medical devices?
For innovators, understanding medical devices is vital as it defines the spectrum of healthcare instruments and highlights their significant potential to enhance patient care.
What is the projected growth of the healthcare equipment sector?
The healthcare equipment sector is projected to reach approximately $678.88 billion by 2025, growing at a compound annual growth rate (CAGR) of 6%.
What factors are driving the growth of the healthcare equipment sector?
The growth is driven by technological advancements and an increasing demand for innovative healthcare solutions, such as AI-powered surgical robots that improve surgical precision and reduce recovery times.
How are medical devices classified?
Medical devices can be classified into various groups, including healthcare tools, diagnostic tools, surgical instruments, and implantable items.
What is the significance of the diagnostic imaging sector in healthcare?
The diagnostic imaging sector accounts for about 20% of the total healthcare equipment market, emphasizing its crucial role in modern healthcare.
How are digital health technologies impacting healthcare?
The integration of digital health technologies, such as telemedicine and wearable devices, is expected to enhance patient monitoring and treatment efficiency, transforming healthcare provision.