


Understanding the critical world of pharmaceutical development reveals a vital yet often overlooked discipline: regulatory affairs. This field is essential in ensuring that drugs are safe, effective, and compliant with stringent regulations before they reach consumers. As pharmaceutical companies navigate a complex landscape of approvals and compliance, one must consider: how do regulatory affairs professionals balance the demands of innovation with the necessity of public safety? Exploring this dynamic uncovers not only the mechanisms behind drug approval but also highlights the evolving challenges faced by the industry in a rapidly changing regulatory environment.
What are regulatory affairs in pharmaceutical companies is a critical discipline that ensures compliance with the regulations governing the development, testing, manufacturing, and marketing of pharmaceutical products. This field, which is fundamentally about what are regulatory affairs in pharmaceutical companies, encompasses a wide range of activities, including the preparation of submissions, communication with oversight agencies, and monitoring adherence to laws and guidelines. Regulatory affairs professionals are essential in safeguarding public health, ensuring that drugs are safe, effective, and of high quality before they reach the market.
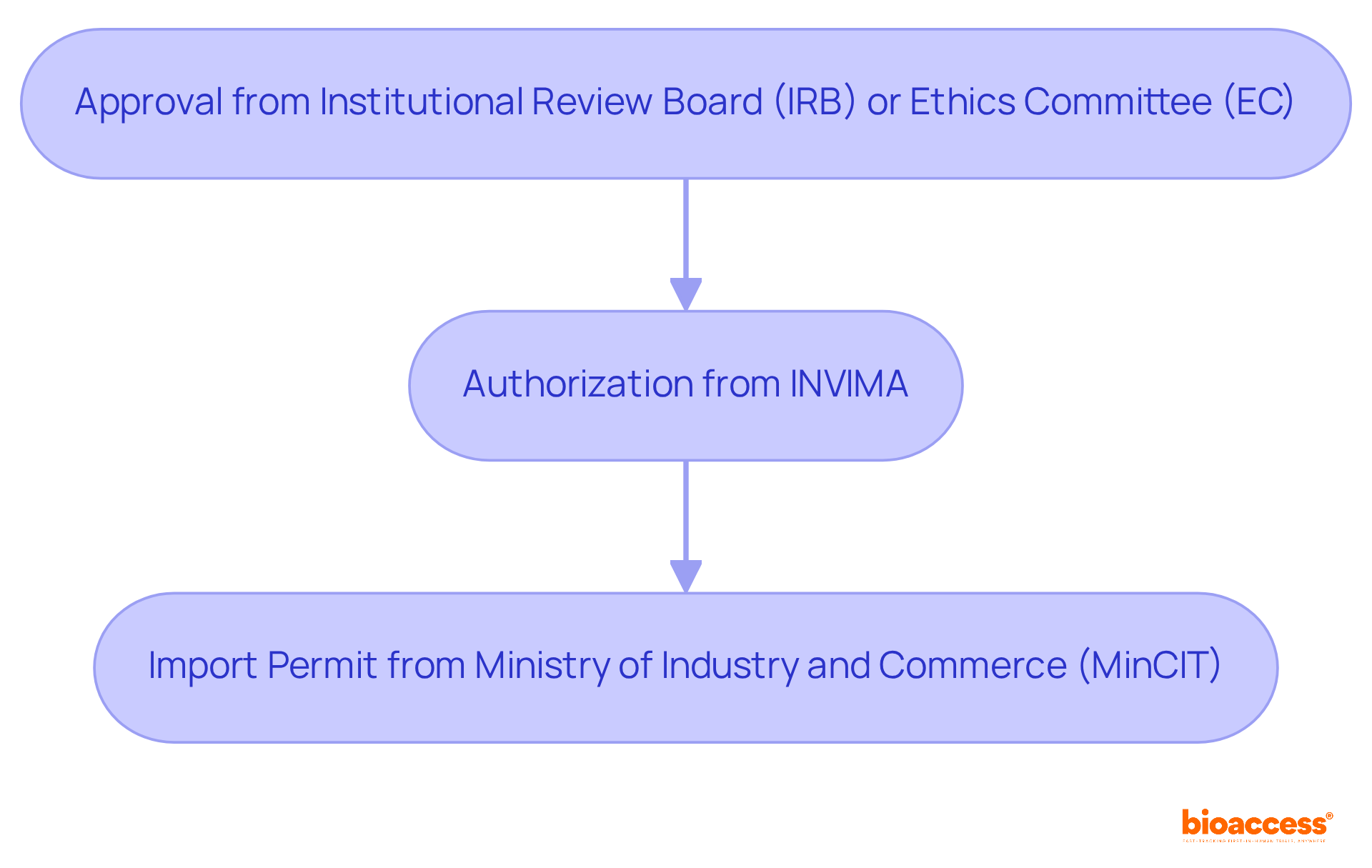
In Colombia, obtaining clinical trial approval involves several key steps:
These steps highlight the complexity of the regulatory landscape and the importance of thorough preparation.
Firms like bioaccess play a significant role in navigating these challenges by offering extensive clinical trial management services. Their offerings include feasibility studies, site selection, compliance evaluations, trial setup, and project oversight. By providing assessment and feedback on study documents and reporting on study progress, bioaccess ensures that clients can efficiently navigate the compliance landscape. This collaboration is vital for the success of clinical research initiatives, ultimately leading to better health outcomes.
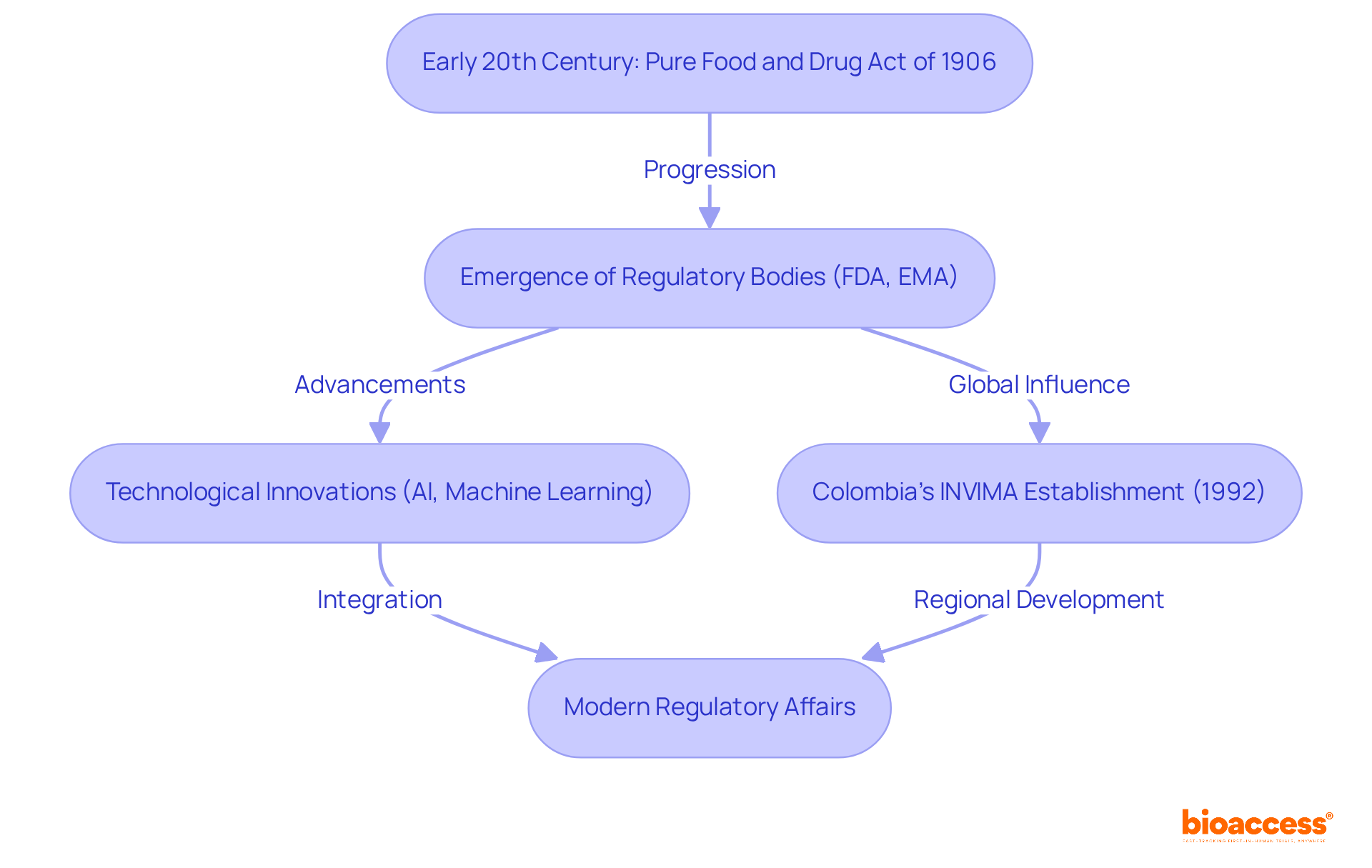
The evolution of what are regulatory affairs in pharmaceutical companies began in the early 20th century, marked by pivotal legislation such as the Pure Food and Drug Act of 1906 in the United States. This landmark action was essential in halting the sale of misbranded or adulterated foods and drugs, laying the groundwork for modern oversight practices. Over the years, this field has adapted to address emerging public health challenges and advancements in science, which raises the question of what are regulatory affairs in pharmaceutical companies, leading to the establishment of regulatory bodies such as the FDA and EMA. These organizations ensure compliance with safety and efficacy standards, which are crucial for public trust.
Today, understanding what are regulatory affairs in pharmaceutical companies is increasingly shaped by technological innovations, including AI and machine learning. These advancements streamline approval processes and enhance patient safety monitoring, making the system more efficient. In Colombia, the INVIMA (Colombia National Food and Drug Surveillance Institute), founded in 1992 under the Ministry of Health and Social Protection, plays a vital role in this regulatory landscape. INVIMA is tasked with inspecting and supervising the marketing and manufacturing of medical products, ensuring compliance with safety standards, and granting medical approval for the import and export of these products.
Specifically, the Directorate for Medical Devices and other Technologies within INVIMA oversees medical devices, recommending technical standards for their manufacturing, marketing, and quality assurance. Notably, INVIMA has been recognized as a Level 4 health authority by the Pan American Health Organization/World Health Organization, highlighting its capability in ensuring the safety, efficacy, and quality of medicines in Colombia. This classification underscores the importance of robust regulatory frameworks in safeguarding public health.
Understanding what are regulatory affairs in pharmaceutical companies is crucial for navigating the complexities of compliance in the Medtech landscape.
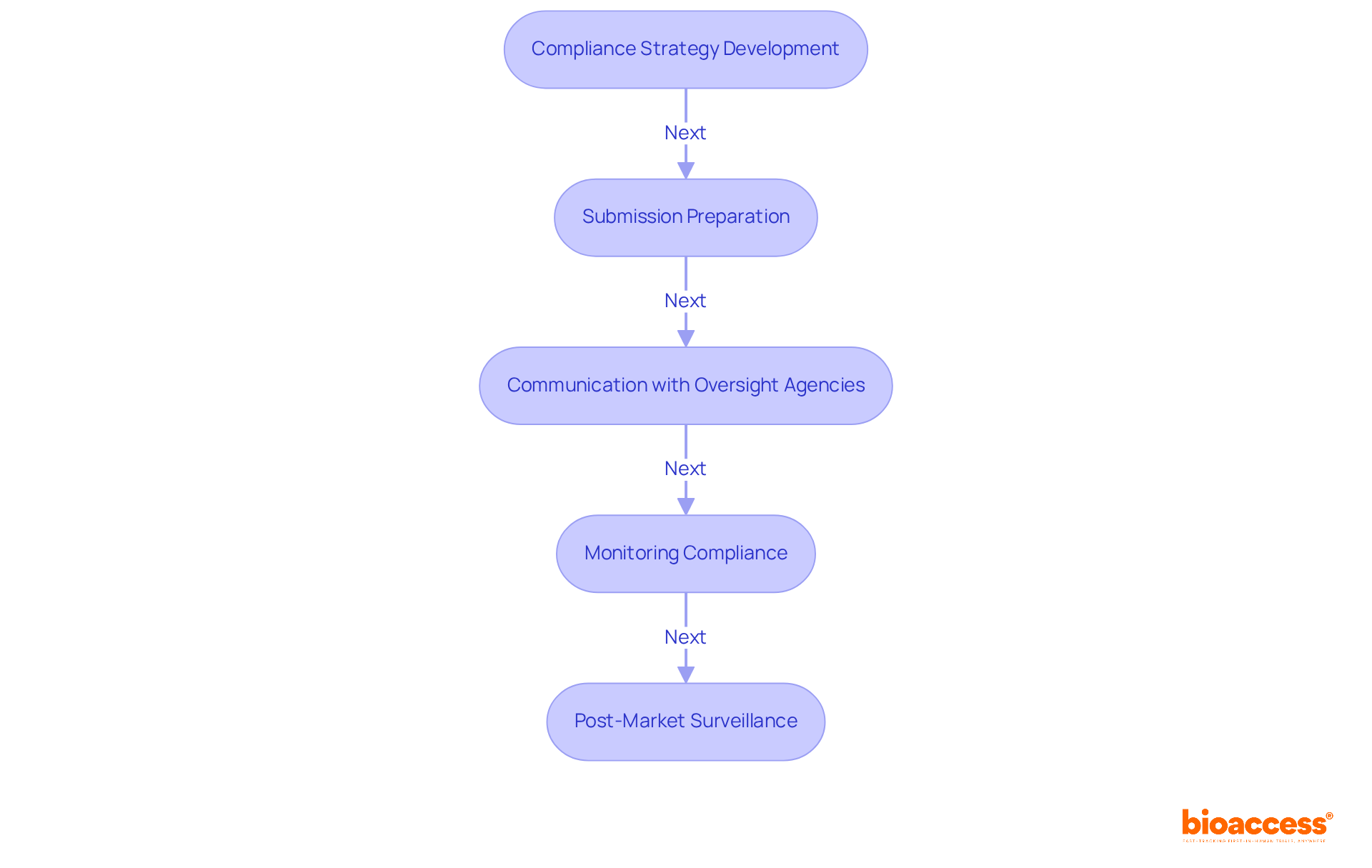
Compliance Strategy Development: Crafting dynamic strategies to navigate the complex oversight landscape is essential for ensuring adherence to both local and international regulations. A well-planned oversight strategy not only secures funding from investors but also aligns with evolving standards. In Latin America, understanding unique compliance requirements and market access challenges is vital for Medtech companies aiming for success in this region.
Submission Preparation: This involves compiling and submitting comprehensive documentation required for official approval, including Investigational New Drug (IND) applications and New Drug Applications (NDA). Firms with robust compliance intelligence often achieve quicker submission timelines, significantly reducing delays. Bioaccess® plays a pivotal role here, connecting innovative Medtech, Biopharma, and Radiopharma startups with top-ranked clinical research sites in Latin America, facilitating faster market access.
Communication with Oversight Agencies: Acting as the intermediary between pharmaceutical companies and oversight bodies, compliance affairs professionals facilitate discussions and clarifications, ensuring alignment on expectations and requirements. This is particularly significant in Latin America, where each nation has its own governing structure and healthcare system.
Monitoring Compliance: Ongoing supervision is essential to guarantee that all elements of medication development conform to official standards throughout the product lifecycle. Companies that actively monitor compliance can avoid costly mistakes and align their operations with regulatory expectations. In Latin America, where market dynamics can shift rapidly, maintaining compliance is crucial for sustained success.
Post-Market Surveillance: This function involves overseeing the monitoring of medications after they are available to ensure ongoing compliance and safety. Effective post-market monitoring is becoming increasingly important, particularly under new regulations that mandate more stringent tracking of adverse reactions and labeling modifications.
Successful instances of submission preparation can be observed in companies like Newleos Therapeutics, which is currently progressing several treatments through human testing. This showcases the significance of strategic submission processes in achieving prompt market entry. Creating a compliance strategy for medication approval requires understanding what are regulatory affairs in pharmaceutical companies, alongside comprehending the distinct criteria of each governing entity and adapting to shifts in the oversight landscape, which is essential for sustaining a competitive advantage in the pharmaceutical sector.
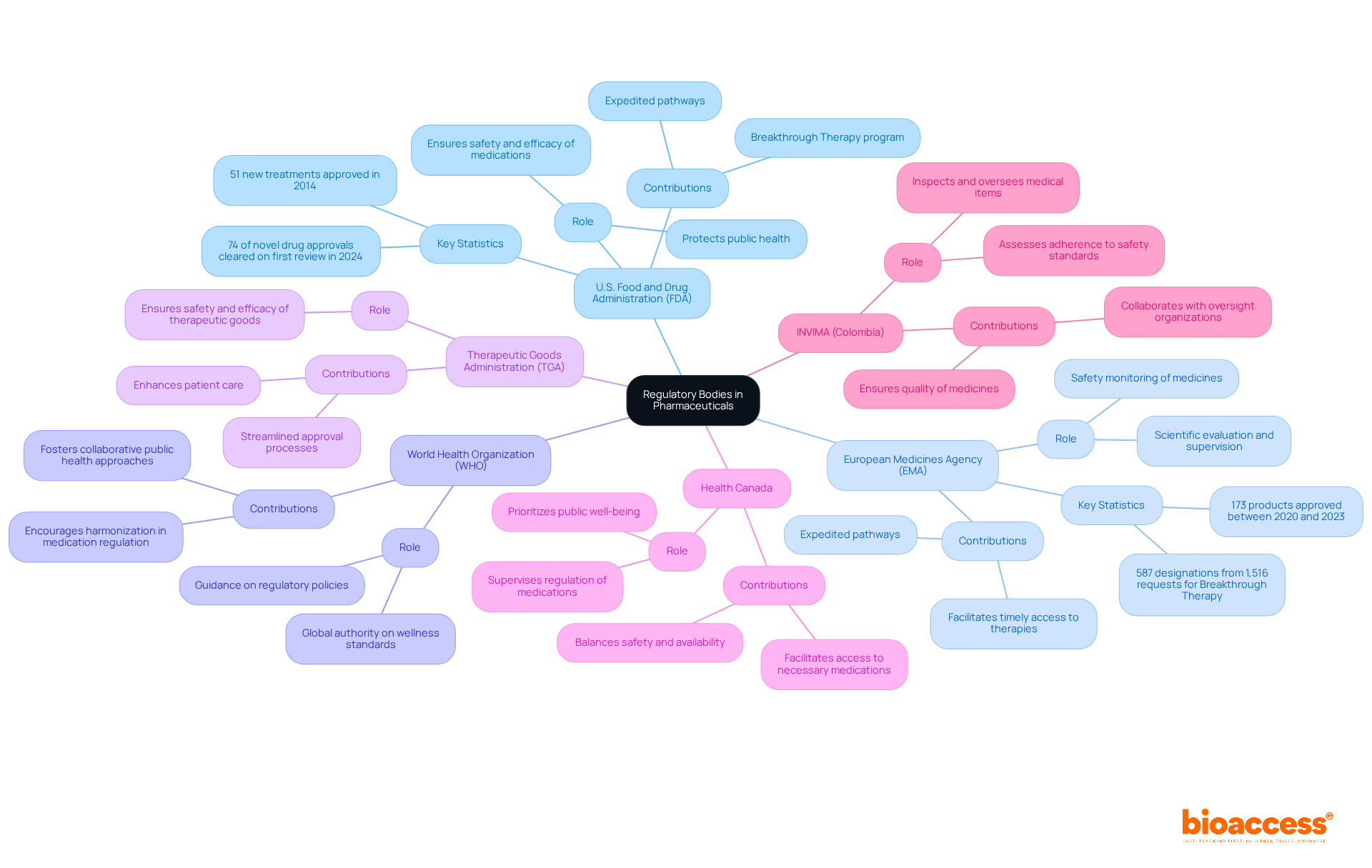
Key regulatory bodies in the pharmaceutical landscape are integral in defining what are regulatory affairs in pharmaceutical companies to ensure the safety and efficacy of medications. For anyone involved in clinical research, understanding what are regulatory affairs in pharmaceutical companies is crucial.
U.S. Food and Drug Administration (FDA): This agency is essential in protecting public health by ensuring that medications, biologics, and medical devices are safe and effective for use in the United States. The FDA's rigorous approval processes have led to a remarkable 74% of novel drug approvals being cleared on the first review in 2024, showcasing its efficiency and commitment to public safety.
European Medicines Agency (EMA): The EMA is pivotal in the scientific evaluation, supervision, and safety monitoring of medicines within the European Union. Between 2020 and 2023, the EMA approved 173 products, reflecting its significant influence on pharmaceutical regulation in Europe. Its expedited pathways, such as the Breakthrough Therapy program, have granted 587 designations from 1,516 requests, demonstrating a strong commitment to facilitating timely access to innovative therapies.
World Health Organization (WHO): As a global authority, the WHO provides essential guidance on wellness standards and practices, shaping regulatory policies worldwide. Its influence encourages harmonization in medication regulation and safety across multiple nations, fostering a collaborative approach to public health.
Therapeutic Goods Administration (TGA): In Australia, the TGA ensures that therapeutic goods meet stringent safety and efficacy standards. The agency's streamlined approval processes contribute to the rapid availability of new treatments in the Australian market, enhancing patient care.
Health Canada: This agency supervises the regulation of medications and wellness items in Canada, prioritizing public well-being. Health Canada’s regulatory framework is designed to protect Canadians while facilitating access to necessary medications, ensuring a balance between safety and availability.
INVIMA (Colombia National Food and Drug Surveillance Institute): Founded in 1992 under Colombia's Ministry of Health and Social Protection, INVIMA is tasked with inspecting and overseeing the marketing and production of medical items. It assesses adherence to safety standards and applies optimal methods for the import and export of goods. The Directorate for Medical Devices and other Technologies within INVIMA oversees medical device regulation, ensuring safety and efficacy through pre- and post-market monitoring. Recognized as a Level 4 health authority by the Pan American Health Organization/World Health Organization, INVIMA plays a critical role in guaranteeing the quality of medicines in Colombia. It collaborates with other oversight organizations to enhance the overall governance framework, ensuring thorough supervision of pharmaceutical items.
Each of these organizations significantly influences the oversight framework, which is essential for understanding what are regulatory affairs in pharmaceutical companies, ensuring that pharmaceutical products are safe for public use. Their collaborative efforts and regulatory frameworks impact drug safety and efficacy, ultimately enhancing patient care across the globe.
Regulatory affairs in pharmaceutical companies stand as a cornerstone in ensuring that medications are developed, tested, and marketed in compliance with stringent regulations. This critical discipline not only protects public health by guaranteeing the safety and efficacy of drugs but also facilitates the complex processes that govern pharmaceutical innovation. Understanding and navigating the regulatory landscape is essential for any entity involved in the pharmaceutical industry.
The article highlights several key aspects of regulatory affairs, including:
It underscores the importance of compliance strategies, submission preparation, and ongoing monitoring to ensure that pharmaceutical products meet the necessary safety standards. Moreover, the discussion of INVIMA's role in Colombia exemplifies how local regulations can shape the pharmaceutical landscape and impact market access.
In light of the complexities surrounding drug approval and the rapid advancements in technology, the significance of regulatory affairs cannot be overstated. As the pharmaceutical industry continues to evolve, stakeholders must remain vigilant in understanding regulatory requirements and adapting to changes. Engaging with regulatory affairs not only enhances compliance but also fosters innovation, ultimately leading to improved health outcomes and patient safety. Embracing this discipline is essential for any organization aiming to thrive in the competitive pharmaceutical landscape.
What are regulatory affairs in pharmaceuticals?
Regulatory affairs in pharmaceuticals is a critical discipline that ensures compliance with regulations governing the development, testing, manufacturing, and marketing of pharmaceutical products. It involves activities such as preparing submissions, communicating with oversight agencies, and monitoring adherence to laws and guidelines.
Why are regulatory affairs professionals important?
Regulatory affairs professionals are essential in safeguarding public health by ensuring that drugs are safe, effective, and of high quality before they reach the market.
What are the key steps to obtain clinical trial approval in Colombia?
In Colombia, obtaining clinical trial approval involves securing approval from the site's institutional review board (IRB) or ethics committee (EC), obtaining authorization from INVIMA (the National Food and Drug Surveillance Institute), and acquiring an import permit from the Ministry of Industry and Commerce (MinCIT) for investigational devices.
What role do firms like bioaccess play in the regulatory landscape?
Firms like bioaccess play a significant role in navigating regulatory challenges by offering extensive clinical trial management services, including feasibility studies, site selection, compliance evaluations, trial setup, and project oversight. They help clients efficiently navigate the compliance landscape, which is vital for the success of clinical research initiatives.
How do regulatory affairs contribute to better health outcomes?
By ensuring that clinical research initiatives comply with regulations and guidelines, regulatory affairs contribute to better health outcomes by facilitating the development and availability of safe and effective pharmaceutical products.