


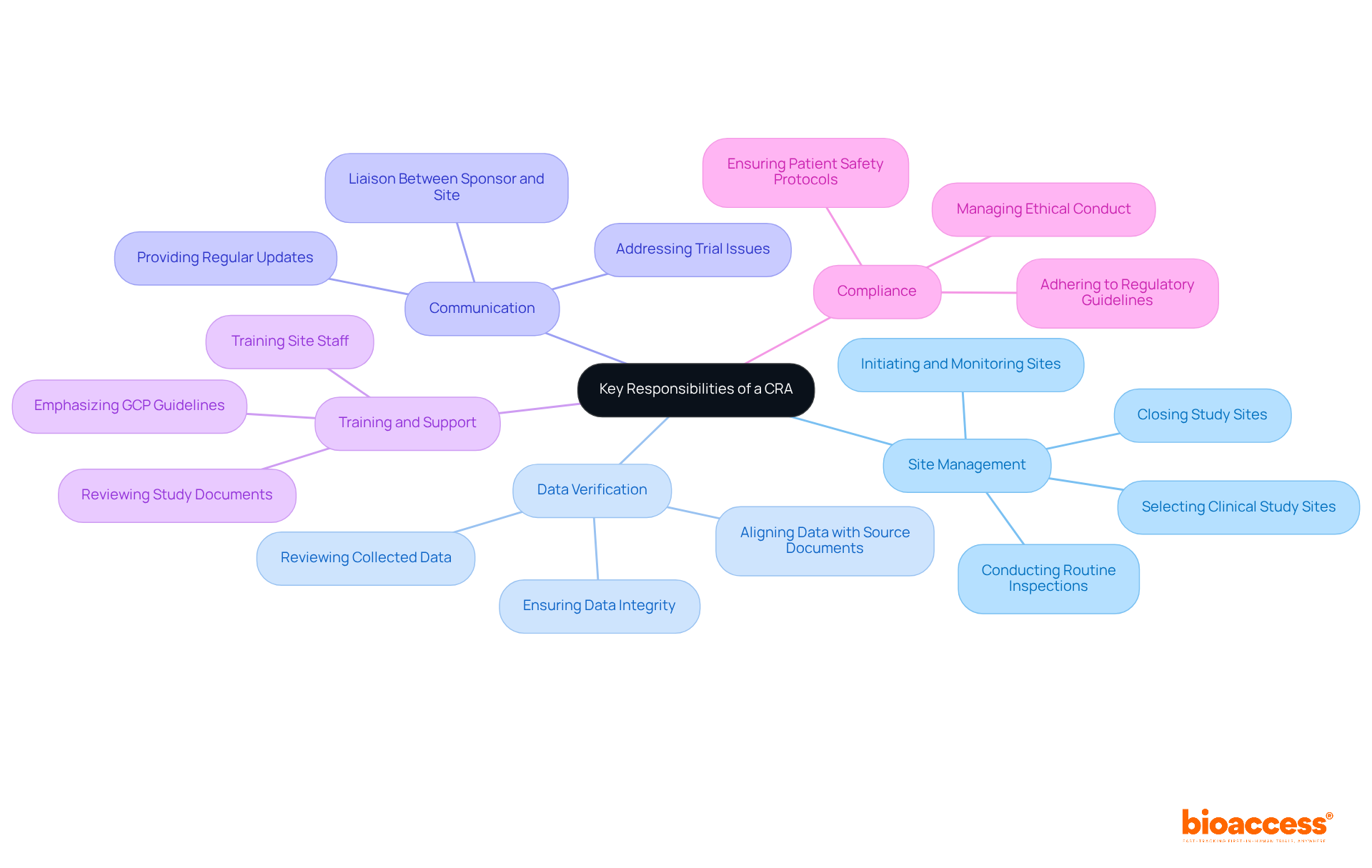
The primary role of a Clinical Research Associate (CRA) is to oversee clinical studies, ensuring compliance with regulatory requirements and Good Clinical Practice (GCP) guidelines to safeguard patient safety and data integrity. This role is crucial in the clinical research landscape, where CRAs serve as vital intermediaries between study sponsors and clinical sites. Key responsibilities include:
All of which are essential for the advancement of medical research. By fulfilling these duties, CRAs not only uphold the integrity of clinical trials but also contribute significantly to the overall progress of medical science.
A Clinical Research Associate (CRA) plays a pivotal role in the intricate realm of clinical trials, acting as a vital link that ensures ethical research practices and adherence to stringent regulations. As the landscape of medical research continues to evolve, it is essential to grasp the multifaceted responsibilities of a CRA to understand the development and testing of new therapies.
What challenges do CRAs encounter in safeguarding data integrity and ensuring patient safety amid rapid technological advancements and shifting regulations? Delving into these inquiries uncovers the indispensable contributions of CRAs to the future trajectory of clinical research.
A Clinical Research Associate (CRA) plays a pivotal role in supervising clinical studies, prompting the question: what does a CRA do as a vital intermediary between study sponsors and clinical locations? Their primary responsibility, which encompasses what does a CRA do, is to ensure that trials comply with regulatory requirements and Good Clinical Practice (GCP) guidelines. In 2025, the contributions of clinical research associates to regulatory compliance and patient safety are increasingly recognized, with approximately 70% of their activities focused on these areas. This emphasis on adherence is crucial, as it directly impacts the integrity of medical research and the safety of participants.
CRAs oversee progress, validate data precision, and ensure that patient safety protocols are rigorously followed during research. Their involvement is essential in maintaining the integrity of clinical research, facilitating the advancement of new medical therapies, and ensuring that studies meet ethical standards. They are integral to the comprehensive process of conducting medical device trials, which includes:
For instance, a recent case study illustrated how a CRA's diligent oversight led to the identification and correction of a data discrepancy that could have jeopardized patient safety, highlighting the significance of their role.
Industry leaders assert that clinical research associates are not merely monitors but also advocates for patient safety and regulatory adherence. As one expert noted, "The CRA's role is essential in closing the divide between compliance and patient welfare, ensuring that every study is conducted with the utmost integrity."
In summary, a CRA's role, or what does a CRA do, is indispensable to the research process, with responsibilities that extend beyond monitoring to encompass a commitment to ethical standards and patient safety. Their expertise and attentiveness, along with critical qualities such as communication skills and meticulousness, are vital for the successful execution of medical studies, ultimately contributing to the advancement of medical science.

The key responsibilities of a Clinical Research Associate (CRA) include understanding what does a CRA do, which encompasses several critical functions essential for the success of clinical trials.
Site Management: CRAs are tasked with selecting, initiating, monitoring, and closing clinical study sites. They conduct routine site inspections, averaging several per experiment, to ensure adherence to the protocol and regulatory requirements. Understanding what does a CRA do is vital for preserving the integrity of the experiment. Bioaccess aids this process by offering feasibility studies and assisting in the selection of research locations and principal investigators (PIs), ensuring that clinical research associates have the necessary resources and information at their disposal.
A crucial aspect of what does a CRA do involves reviewing and verifying the accuracy of data collected during trials. This process ensures alignment with source documents, safeguarding data integrity and reliability. Contract Research Associates typically spend an average of 165 hours each month managing their responsibilities, which includes this essential verification process. Bioaccess enhances this by providing comprehensive project management services that optimize data collection and reporting, helping to clarify what does a CRA do to ensure data integrity.
Communication: Acting as the primary liaison between the sponsor and the clinical site, CRAs exemplify what does a CRA do by facilitating effective communication. They address any issues that arise during the trial, ensuring that all parties remain informed and aligned. As Kenneth A. Getz, Senior Research Fellow at the Tufts CSDD, observes, "Ultimately, more informed CRA management better ensures data quality and integrity, the safe and ethical treatment of research participants, protocol adherence, and more successful site performance." Bioaccess supports this communication by offering regular updates on research status and inventory, aiding clinical research associates in maintaining alignment with all stakeholders.
Training and Support: CRAs provide essential training to site staff on the study protocol and procedures. This training ensures that everyone involved understands what does a CRA do, along with their roles and responsibilities, which is critical for the smooth operation of the trial. Emphasizing Good Clinical Practice (GCP) guidelines during training is vital for maintaining high standards in clinical research. Bioaccess contributes by reviewing and providing feedback on study documents to ensure compliance with country requirements, thereby enhancing the training process.
What does a CRA do? Ensuring adherence to regulatory guidelines is a fundamental responsibility of Clinical Research Associates. They manage all elements of the study to comply with ethical guidelines and patient safety protocols, thereby maintaining the highest standards of medical research. The importance of compliance is underscored by the evolving regulatory landscape, which demands rigorous adherence to GCP guidelines. Bioaccess expedites approval procedures for startups by managing study setup, initiation, acquiring essential import permits, and nationalizing investigational devices, thus alleviating some of the regulatory challenges Clinical Research Associates encounter.
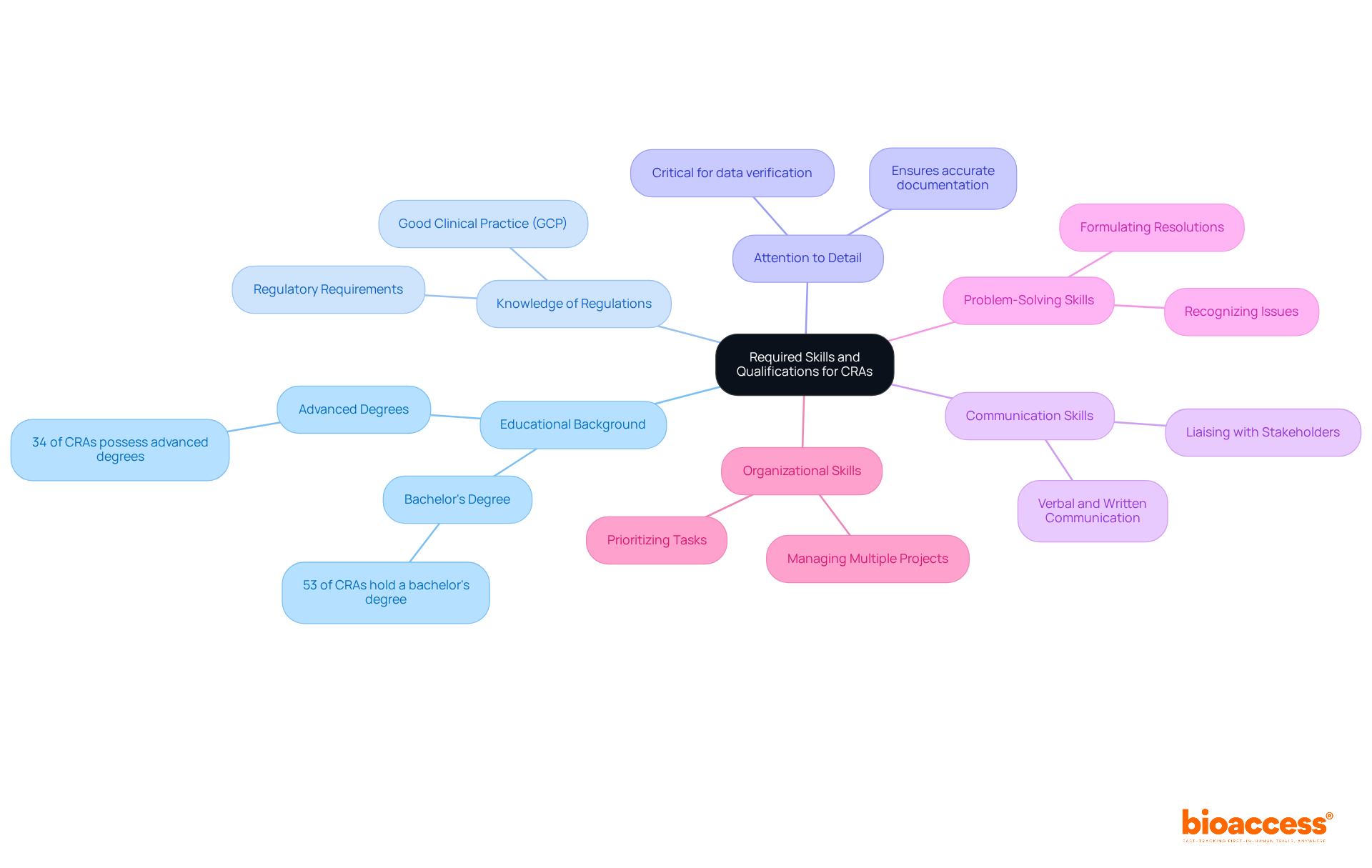
To excel as a Clinical Research Associate (CRA), candidates must understand what does a CRA do and possess a combination of skills and qualifications that align with industry demands, especially in the area of comprehensive clinical trial management services provided by organizations like Bioaccess.
Educational Background: A bachelor's degree in life sciences, nursing, or a related field is often a prerequisite. Approximately 53% of CRAs hold a bachelor's degree, while over 34% possess advanced degrees—significantly enhancing job prospects and earning potential.
Knowledge of Regulations: A solid comprehension of Good Clinical Practice (GCP), regulatory requirements, and ethical guidelines is essential for ensuring adherence throughout clinical studies. This knowledge is crucial for preserving the integrity of the research process, especially when navigating the complexities of study setup, compliance reviews, and import permits as part of Bioaccess's services.
Attention to Detail: CRAs must exhibit exceptional attention to detail to accurately verify data and ensure meticulous documentation of all trial activities. This skill is vital for the success of clinical research, as even minor mistakes can lead to significant issues, particularly in documenting serious and non-serious adverse events.
Communication Skills: Strong verbal and written communication abilities are essential for effectively liaising with study sponsors, site staff, and regulatory bodies. Effective communication fosters collaboration and ensures that all stakeholders remain informed and aligned—critical during the setup and monitoring phases.
Problem-Solving Skills: Clinical research associates should be adept at recognizing issues that arise during studies and formulating prompt resolutions. This proactive approach is vital for sustaining the momentum of clinical studies and addressing challenges before they escalate, particularly in the context of project management and monitoring.
Organizational Skills: The capacity to manage multiple projects and timelines is essential, as clinical research associates often oversee several studies concurrently. Strong organizational abilities enable CRAs to prioritize tasks efficiently, ensuring that all aspects of the studies are executed seamlessly, in alignment with the extensive services offered by Bioaccess.
In summary, knowing what does a CRA do, along with a solid educational foundation, regulatory knowledge, and essential soft skills, equips research associates for success in a rapidly evolving environment, particularly within the framework of accelerated research services for medical devices.
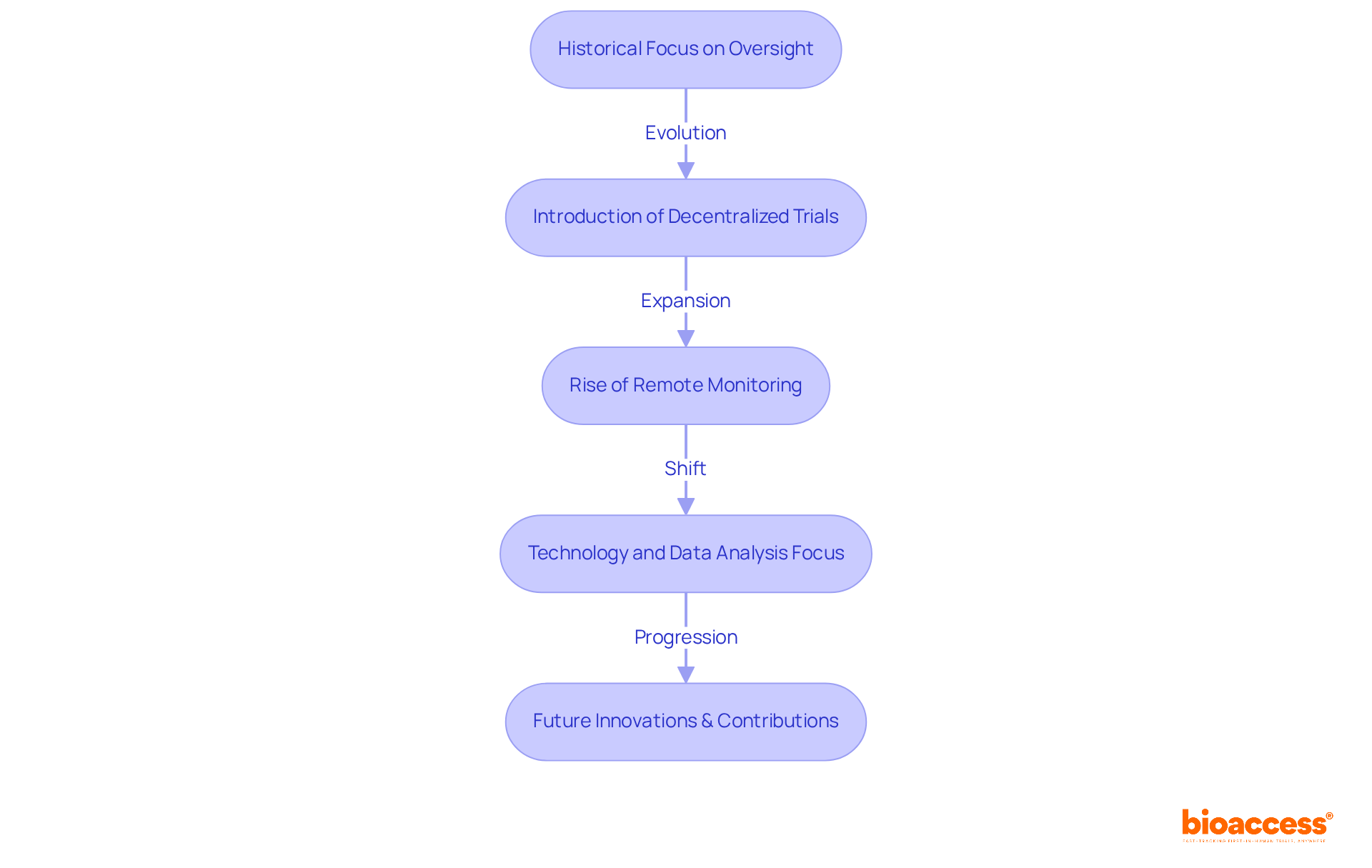
The transformation in the function of Clinical Research Associates raises the question of what does a CRA do, as it has been significantly influenced by technological advancements, regulatory changes, and the growing complexity of clinical studies. Historically, these professionals prioritized oversight and data validation. However, the emergence of decentralized trials and remote monitoring technologies has expanded their role to include strategic oversight and improved collaboration with site staff, leading to the inquiry of what does a CRA do.
For instance, during the pandemic, an impressive 76% of Clinical Research Associates conducted monitoring visits remotely, a notable increase from just 18% in 2019. This evolution necessitated a focus on technology and data analysis, leading to a better understanding of what does a CRA do to streamline processes and boost efficiency.
As the medical research landscape continues to evolve, these professionals are expected to adopt innovative methods and technologies, solidifying their role as vital contributors to the future of research trials. With the integration of digital tools, Clinical Research Associates can enhance data collection and analysis, ensuring they remain at the forefront of innovation in clinical research.
The role of a Clinical Research Associate (CRA) is essential in ensuring the integrity and compliance of clinical trials, acting as a crucial link between study sponsors and clinical sites. Understanding what a CRA does goes beyond mere oversight; it encompasses a commitment to regulatory adherence, patient safety, and ethical standards fundamental to advancing medical research.
Throughout this discussion, the key responsibilities of CRAs have been highlighted, including:
These functions are vital for maintaining high standards in clinical trials, ensuring that all processes align with Good Clinical Practice (GCP) guidelines. The evolution of the CRA role, particularly in response to technological advancements and the complexities of modern clinical studies, underscores the importance of adaptability and continuous learning in this profession.
As the landscape of clinical research continues to change, the demand for skilled CRAs who can navigate these challenges will only grow. Emphasizing the significance of their role not only reinforces the value of CRAs in safeguarding patient welfare but also invites aspiring professionals to consider the impactful career opportunities within clinical research. The future of medical innovation relies on the expertise and dedication of Clinical Research Associates, making their contributions more crucial than ever.
What is the primary role of a Clinical Research Associate (CRA)?
The primary role of a CRA is to supervise clinical studies and ensure that trials comply with regulatory requirements and Good Clinical Practice (GCP) guidelines.
What percentage of a CRA's activities focus on regulatory compliance and patient safety?
Approximately 70% of a CRA's activities focus on regulatory compliance and patient safety.
What are some key responsibilities of a CRA?
Key responsibilities of a CRA include overseeing progress, validating data precision, ensuring patient safety protocols are followed, conducting feasibility evaluations, site selection, compliance reviews, trial setup, managing projects, and reporting.
How does a CRA contribute to patient safety during clinical trials?
A CRA contributes to patient safety by rigorously following safety protocols, overseeing the integrity of clinical research, and identifying and correcting discrepancies that could jeopardize patient safety.
Are CRAs only responsible for monitoring clinical trials?
No, CRAs are not merely monitors; they are also advocates for patient safety and regulatory adherence, ensuring that studies are conducted with integrity.
What qualities are important for a successful CRA?
Important qualities for a successful CRA include strong communication skills, meticulousness, and attentiveness.
How do CRAs impact the advancement of medical therapies?
CRAs maintain the integrity of clinical research, which facilitates the advancement of new medical therapies by ensuring ethical standards and regulatory compliance are met.