


Monoclonal antibodies (mAbs) are laboratory-engineered proteins designed to replicate the immune system's ability to target specific pathogens. They play a crucial role in treating diseases such as cancer, autoimmune disorders, and infections. This overview highlights their development history, therapeutic applications, and the ongoing challenges in production and immunogenicity. The significance of mAbs in modern medicine cannot be overstated, as they represent a promising frontier for future advancements in the field.
Monoclonal antibodies, or mAbs, signify a remarkable leap forward in medical science, presenting targeted therapies that transform treatment for a variety of diseases. These laboratory-engineered proteins are meticulously designed to emulate the immune system's capacity to fight harmful pathogens, establishing their critical role in fields such as oncology and immunology.
However, as the mAb development landscape continues to evolve, notable challenges remain, prompting inquiries about their future applications and effectiveness. What implications do these innovations hold, and how might they influence the future of clinical research and patient care?
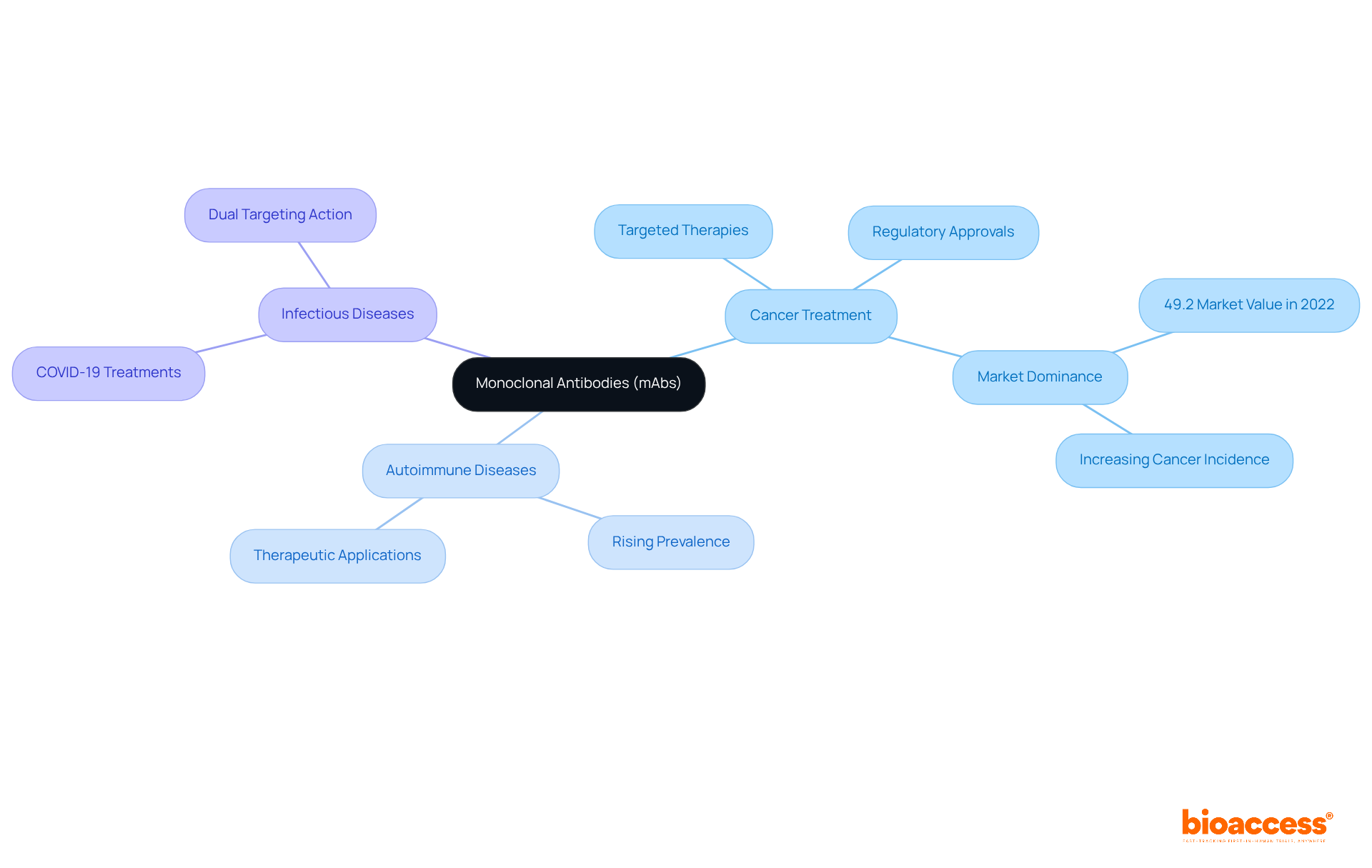
Monoclonal proteins, commonly referred to as mAbs, are laboratory-engineered proteins designed to emulate the immune system's ability to fight off harmful pathogens. These proteins are produced from a single clone of B cells, resulting in uniform proteins that specifically target a particular antigen. This level of specificity allows monoclonal antibodies to be employed in a wide array of therapeutic contexts, including:
For professionals in the medical and research sectors, understanding what mAbs stand for is essential, as it signifies a pivotal advancement in the realm of targeted therapies.
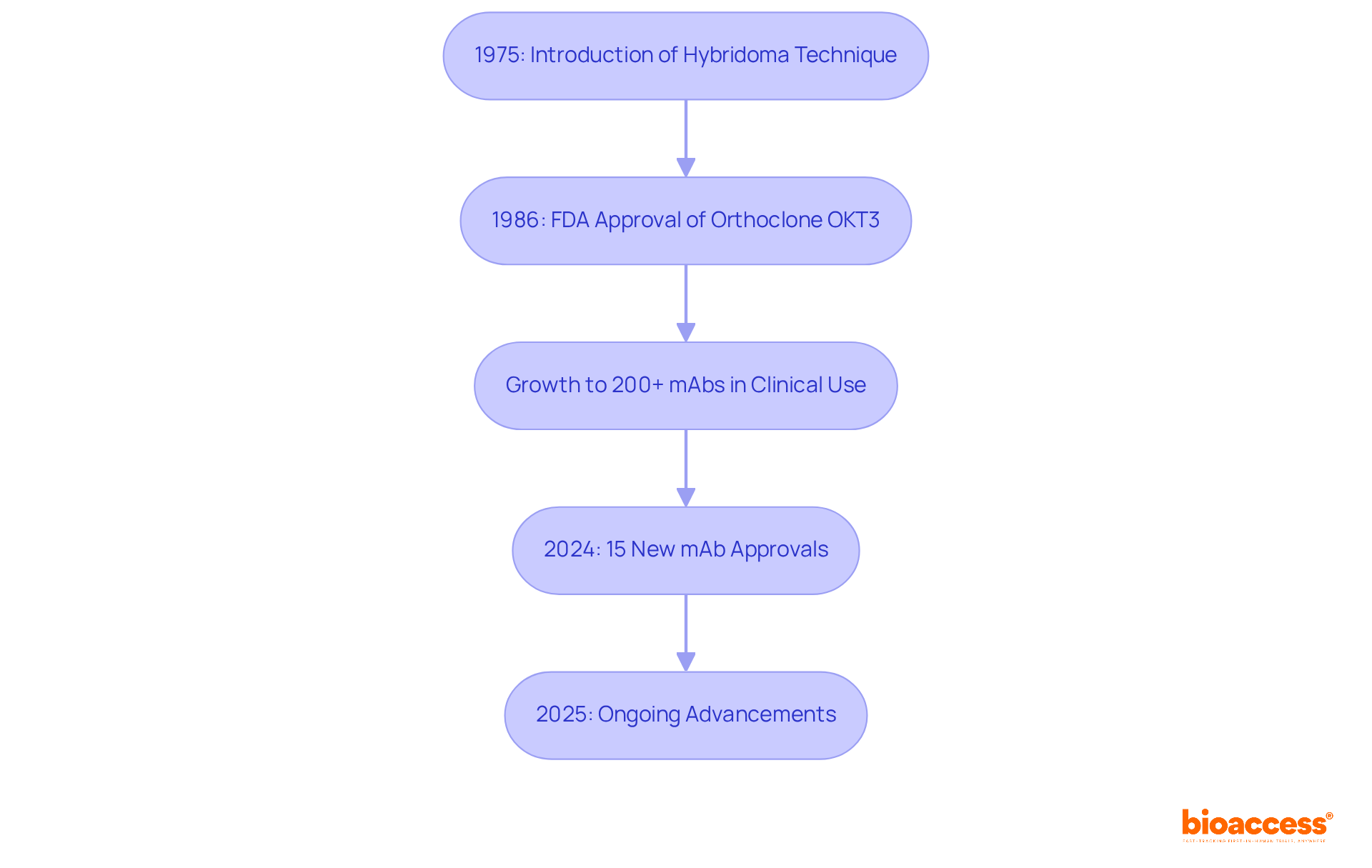
The concept of monoclonal antibodies (mAbs) originated in 1975, thanks to the pioneering work of Georges Köhler and César Milstein, who introduced the hybridoma technique. This groundbreaking method facilitated the creation of identical antibodies by merging myeloma cells with B cells, resulting in a hybrid cell line capable of indefinite culture.
The first mAb, Orthoclone OKT3, received FDA approval in 1986 for the prevention of kidney transplant rejection, marking a pivotal milestone in therapeutic development. Since that time, the field has witnessed extraordinary growth, with over 200 monoclonal antibodies currently in clinical use, fundamentally altering treatment paradigms across various diseases, especially in oncology and immunology.
As we look toward 2025, the FDA continues to broaden its list of approved monoclonal antibodies, reflecting ongoing advancements in this crucial area of medical research. Notably, in 2024 alone, 15 new antibody-based biologics were approved, underscoring the dynamic nature of this field.
As Stefan Dübel aptly noted, 'Recombinant immunoglobulin technology has transformed the creation of immune system treatments,' highlighting the continuous advancement and significance of monoclonal antibodies in contemporary healthcare.
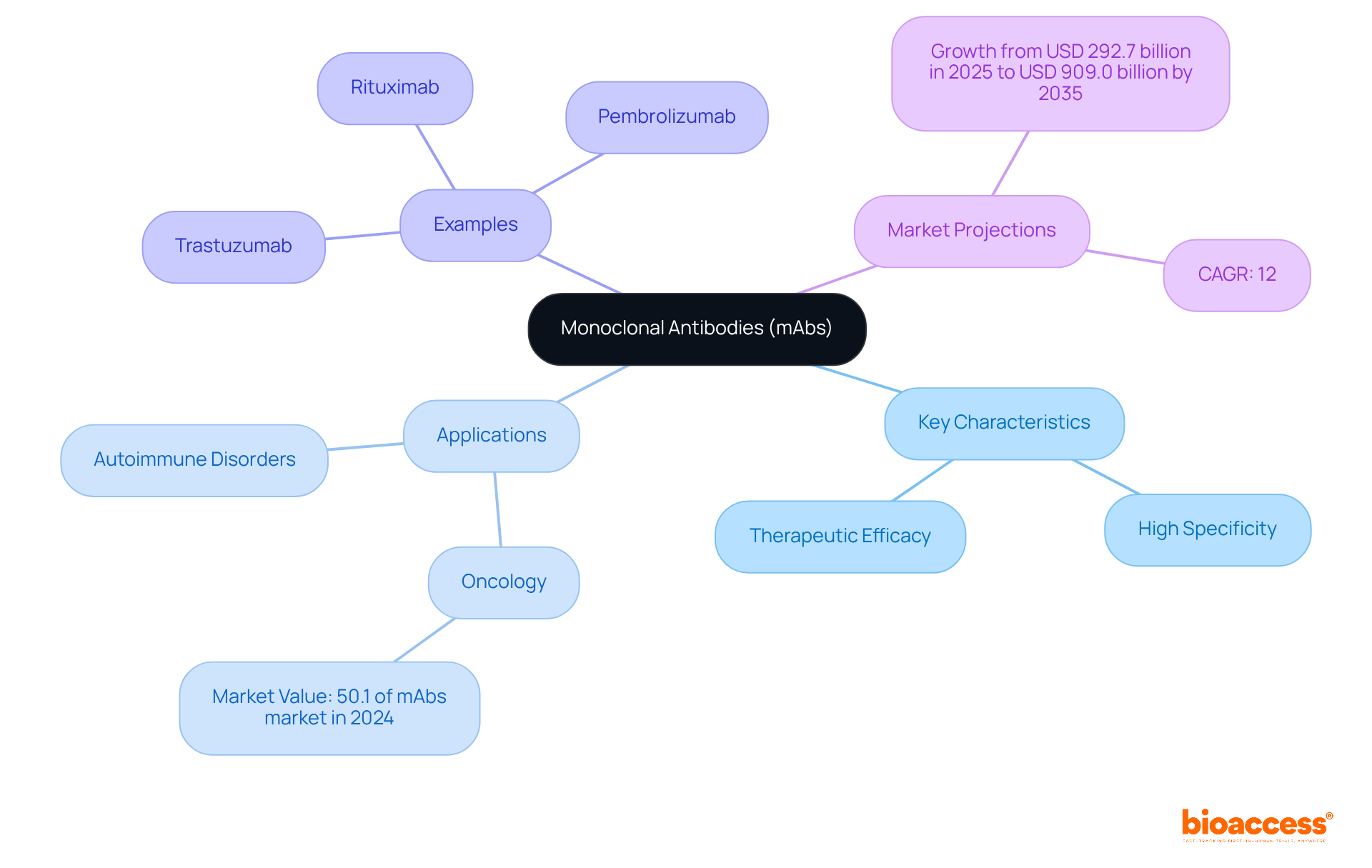
Monoclonal proteins are distinguished by their high specificity for target antigens, significantly reducing off-target effects and enhancing therapeutic efficacy. This precision is crucial in contemporary medicine, particularly in targeted cancer treatments, where monoclonal antibodies can effectively identify and eliminate cancer cells through immune system activation. Notably, the oncology application segment represented 50.1% of the monoclonal antibodies market value in 2024, spurred by numerous regulatory approvals for cancer therapies. Furthermore, monoclonal antibodies are increasingly utilized in managing autoimmune disorders, where they can mitigate harmful immune responses, thereby improving patient outcomes.
The versatility of monoclonal antibodies is further exemplified by their design capabilities, which allow for enhanced characteristics such as extended half-life and modified immune response functions. Prominent examples of therapeutic monoclonal antibodies include:
Each demonstrating substantial efficacy across various clinical scenarios. This adaptability positions monoclonal therapies as a cornerstone of modern therapeutic strategies. The global market for these products is projected to expand from USD 292.7 billion in 2025 to USD 909.0 billion by 2035, reflecting a compound annual growth rate (CAGR) of 12%. This growth underscores the increasing importance of monoclonal antibodies in addressing a range of medical conditions, despite ongoing challenges such as high development costs and access issues.
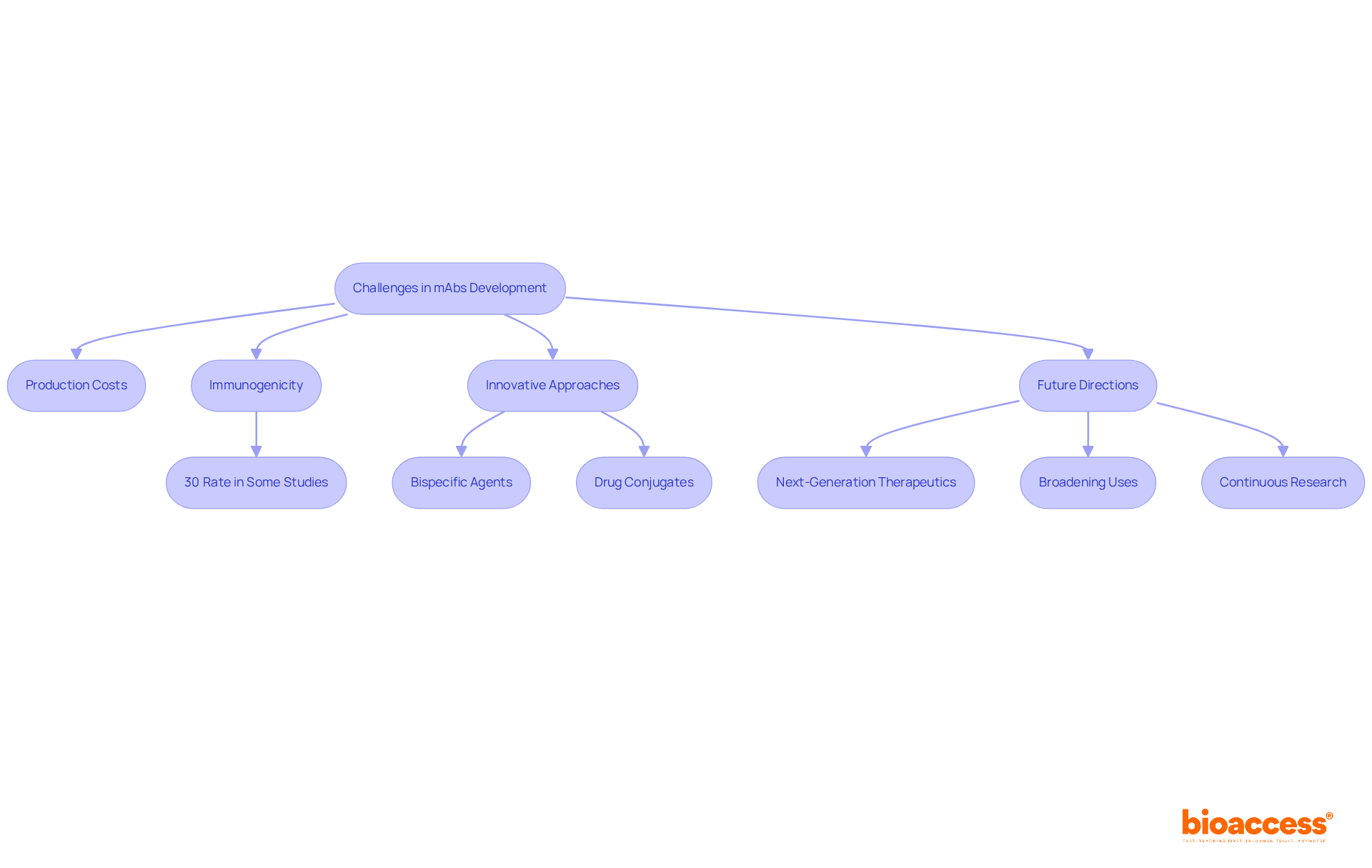
The advancement of monoclonal proteins continues to face considerable hurdles, particularly elevated production expenses and possible immunogenicity, which can affect patient safety and treatment effectiveness. Current statistics indicate that immunogenicity rates in mAbs can vary, with some studies reporting rates as high as 30%. This underscores the pressing need for improved design and testing methodologies. To tackle these challenges, researchers are increasingly adopting innovative approaches, including bispecific agents and drug conjugates, which enhance therapeutic potential by targeting multiple pathways simultaneously.
Advancements in biotechnology, such as phage display and single-cell technologies, are transforming mAb development. These innovations allow for the creation of next-generation therapeutics with improved efficacy and safety profiles. Such technologies facilitate the identification of high-affinity binders and enable more precise engineering of antibodies to minimize adverse immune responses. As the field advances, continuous research is aimed at broadening the uses of monoclonal antibodies beyond cancer and autoimmune disorders, investigating their potential in infectious diseases and tailored medicine.
The future of mAbs appears promising, driven by a commitment to overcoming existing challenges and leveraging cutting-edge technologies. With continued investment in research and development, the goal is to improve patient outcomes and broaden access to these vital therapeutics. This commitment not only addresses current limitations but also paves the way for innovative solutions in clinical research.
Monoclonal antibodies, commonly referred to as mAbs, signify a groundbreaking advancement in targeted medical therapies, poised to transform treatment across a spectrum of diseases. Their unique capacity to specifically target antigens not only enhances efficacy but also minimizes side effects, representing a pivotal shift in the management of conditions such as cancer and autoimmune disorders.
This article delineates the evolution of mAbs, tracing their inception from the hybridoma technique introduced in 1975 to the present day, where over 200 monoclonal antibodies are actively utilized in clinical settings. The key applications in oncology, autoimmune diseases, and infectious diseases highlight their versatility and critical role in contemporary medicine. Moreover, the projected growth of the mAbs market underscores an increasing dependence on these therapies, despite persistent challenges such as elevated production costs and concerns regarding immunogenicity.
As the realm of monoclonal antibodies advances, it is imperative to remain steadfast in overcoming existing challenges while leveraging innovative technologies. By prioritizing research and development, the healthcare community can significantly enhance patient outcomes and expand access to these transformative treatments. Embracing the potential of mAbs not only addresses current limitations but also lays the groundwork for a future where targeted therapies can profoundly elevate the quality of life for patients on a global scale.
What does mAbs stand for?
mAbs stands for monoclonal antibodies, which are laboratory-engineered proteins designed to mimic the immune system's ability to fight off harmful pathogens.
How are monoclonal antibodies produced?
Monoclonal antibodies are produced from a single clone of B cells, resulting in uniform proteins that specifically target a particular antigen.
What are the main applications of monoclonal antibodies?
Monoclonal antibodies are used in various therapeutic contexts, including cancer treatment, autoimmune diseases, and infectious diseases.
Why is understanding mAbs important for medical and research professionals?
Understanding mAbs is essential for professionals in the medical and research sectors as it signifies a pivotal advancement in targeted therapies.