


A case report form (CRF) in clinical trials serves as a standardized document that systematically collects and records essential data from each participant. This process is vital for ensuring consistency and accuracy in the information gathered.
The significance of CRFs cannot be overstated; they are crucial in maintaining data integrity, fulfilling regulatory requirements, and enhancing the quality of research outcomes. Such elements are imperative for the credibility and success of clinical studies, underscoring the indispensable role that CRFs play in the clinical research landscape.
The integrity of clinical trials is fundamentally dependent on the meticulous collection of patient data, a task that is inherently supported by Case Report Forms (CRFs). These specialized documents transcend mere bureaucratic necessities; they are indispensable tools that guarantee consistency, accuracy, and regulatory compliance in research.
As the landscape of clinical research continues to evolve, a pressing challenge emerges: how can researchers effectively leverage CRFs to enhance data quality and streamline the trial process? By exploring the multifaceted role of CRFs, we unveil their critical importance in producing reliable and actionable study outcomes.
In medical studies, a case report form in clinical trials refers to a specialized document utilized to gather information from each participating patient. This standardized tool is crucial for recording all relevant information regarding the patient's health status, treatment, and outcomes during the study.
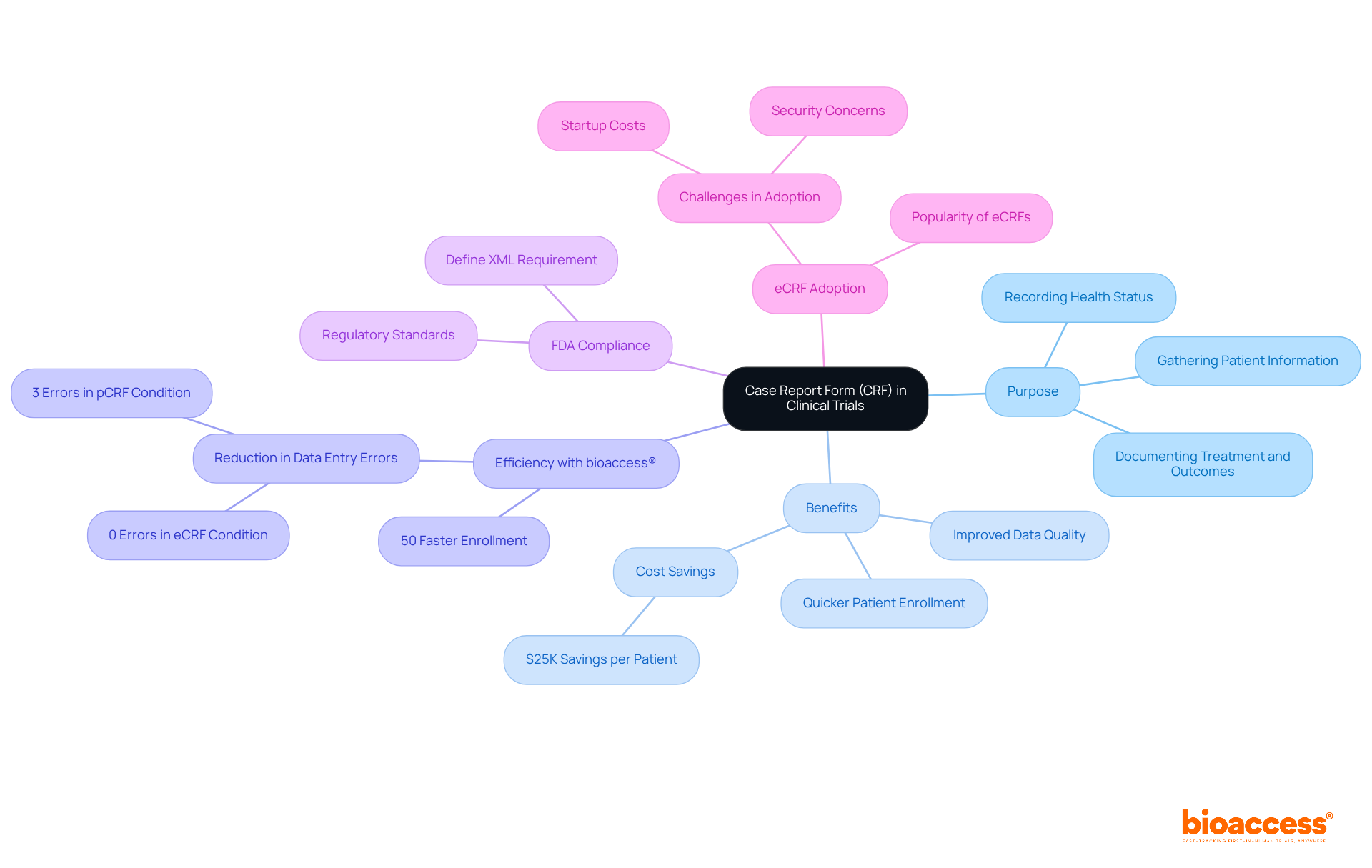
To understand what a case report form in clinical trials is, it is important to know that CRFs ensure that information is collected consistently and accurately, facilitating the analysis and interpretation of results. Notably, with bioaccess®, studies can achieve patient enrollment 50% quicker than conventional Western locations, leading to substantial savings of $25K for each patient.
This efficiency is bolstered by FDA-ready information, which simplifies the CRF process by ensuring that the data gathered meets regulatory standards from the outset, thus eliminating the need for rework and delays. As electronic case report forms (eCRFs) gain popularity due to their efficiency and simplicity in managing information, incorporating bioaccess®'s features can further enhance the research process, effectively addressing the frequent recruitment challenges faced by Medtech and biopharma startups.
In clinical research, understanding what is a case report form in clinical trials is crucial, as Case Report Forms (CRFs) serve multiple critical functions. They standardize information collection across all study locations, which is vital for preserving the integrity of the trial. By ensuring uniformity, CRFs help mitigate discrepancies that could compromise the validity of the results. Moreover, CRFs fulfill regulatory obligations by providing a comprehensive account of the information gathered and the methods used during the research. This documentation is crucial for audits and inspections, reinforcing the transparency and accountability of clinical research processes.
Furthermore, well-organized CRFs significantly improve information quality by decreasing the chances of mistakes and omissions. Research has demonstrated that adopting efficient information quality management systems can enhance accuracy and reliability, with acceptable error rates for essential quality control established at 0%. This focus on quality is evident in the findings that missing data queries accounted for 21.8% of data queries (DQs), underscoring the importance of comprehensive data collection tools.
CRFs also aid in monitoring and auditing, which are essential for ensuring adherence to International Conference on Harmonisation Good Clinical Practice (ICH-GCP) guidelines. These guidelines emphasize the safeguarding of patient rights and the credibility of research outcomes, establishing CRFs as a fundamental element of ethical medical research. An analysis of the intricacies of procedures in medical research indicates that as protocols grow more complex, particularly in oncology, the necessity for robust CRFs becomes increasingly evident to effectively address related quality challenges.
In summary, what is a case report form in clinical trials serves to guarantee information integrity and adherence to regulatory standards while also playing a crucial role in improving the overall quality of research findings. Ultimately, this results in more dependable and actionable study outcomes.

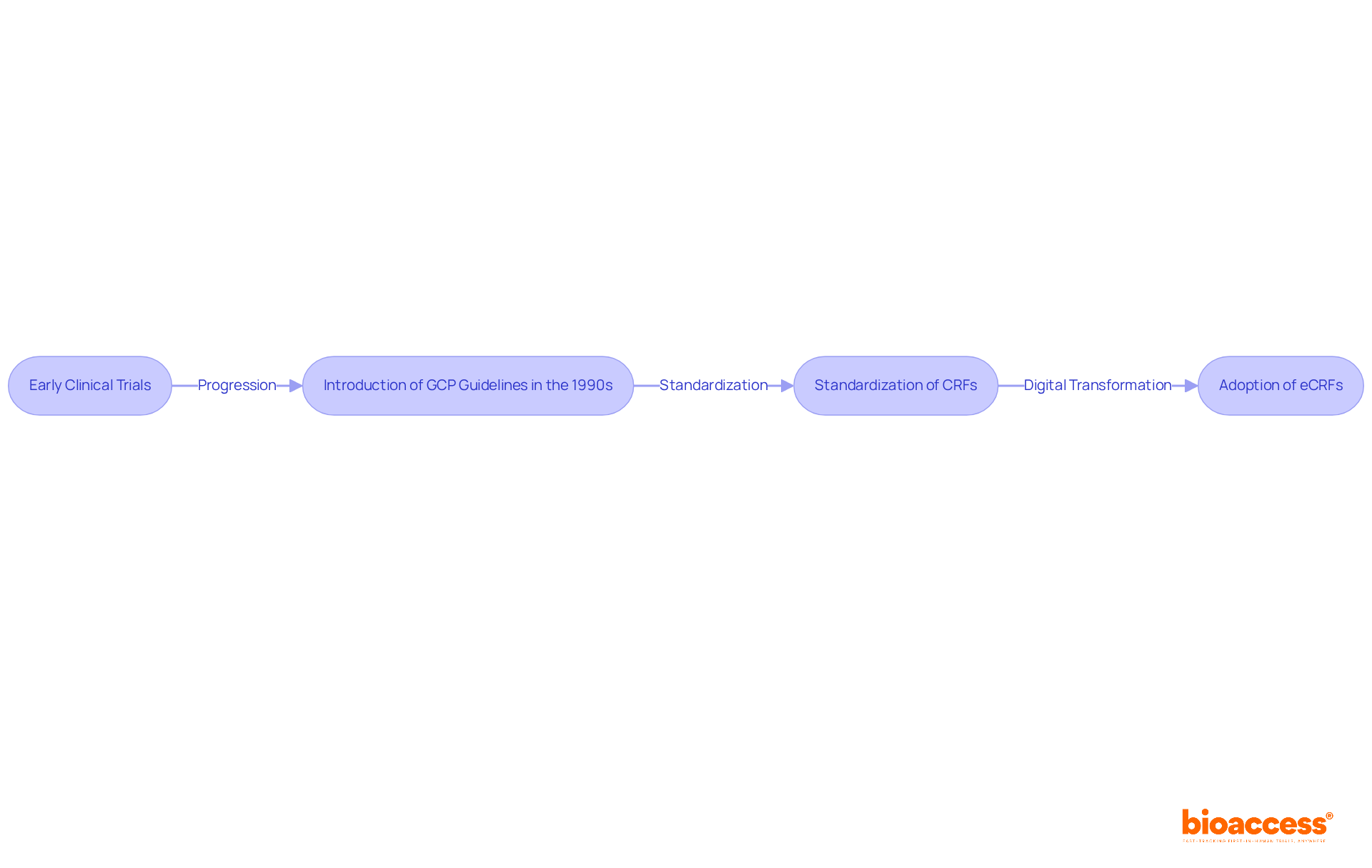
Understanding what is a case report form in clinical trials highlights the evolution from informal and inconsistent information collection methods to standardized tools that are essential for maintaining information integrity. In the early days of clinical trials, the lack of structured information collection led to challenges in analysis and reliability. The introduction of Good Clinical Practice (GCP) guidelines in the 1990s marked a pivotal moment, underscoring the necessity of case report forms for ensuring compliance and enhancing information quality. These guidelines established a framework that mandated detailed documentation, which CRFs are designed to fulfill.
As clinical research progressed, the adoption of electronic Case Report Forms (eCRFs) revolutionized information management. eCRFs facilitate real-time information entry and remote monitoring, significantly enhancing the efficiency of collection processes. A case study examining the effect of direct information entry in a phase 2 migraine treatment study revealed that only 3.7% of over 38,000 forms were queried, with just 2.0% requiring modifications. This statistic highlights the effectiveness of eCRFs in bolstering information integrity. Such efficiency is crucial for organizations like bioaccess®, which provide expert CRO services that optimize data management and participant recruitment, ensuring that studies are conducted effectively.
Today, what is a case report form in clinical trials is not only vital for regulatory adherence but also plays a key role in the overall success of research studies. They ensure that critical information, including participant demographics, medical history, and adverse events, is accurately recorded, thereby contributing to the safety profile of investigational products. The development of case report forms, driven by technological advancements and regulatory needs, underscores their significance in the research environment, particularly as bioaccess® continues to promote expedited studies for medical devices in Latin America.
Understanding what is a case report form in clinical trials involves recognizing the several critical characteristics that effective Case Report Forms (CRFs) possess, which significantly enhance their utility.
Flexibility is another crucial trait; CRFs should be designed to adapt to modifications in study protocols or regulatory demands, ensuring they remain relevant throughout the trial. Incorporating user feedback during the design stage is essential for enhancing the functionality and effectiveness of clinical research forms in practical applications.
Research indicates that user-friendly CRFs can reduce entry mistakes by as much as 5%, while electronic case report forms boast a 0% error rate compared to a 5% error rate for paper forms. Moreover, patients experience a 23% time savings when utilizing electronic CRFs (eCRFs) compared to paper CRFs (pCRFs), underscoring the impact of careful design on information integrity and efficiency.
By focusing on these essential elements, researchers can develop CRFs that not only collect accurate and comprehensive information but also provide clarity on what is a case report form in clinical trials, thereby enhancing the clinical trial process. As Eli Chachak notes, 'Good CRF design focuses not only on the data being captured but also on the experience of the person providing that data.

The significance of case report forms (CRFs) in clinical trials is paramount, as they function as the backbone for collecting and managing essential patient data. By standardizing the documentation process, CRFs guarantee that information is recorded consistently and accurately, which is vital for preserving the integrity of clinical research. This structured approach not only meets regulatory requirements but also enhances the quality of the data collected, leading to more reliable and actionable outcomes.
Key points throughout the article illuminate the multifaceted role of CRFs in clinical research:
The transition from traditional paper forms to electronic case report forms (eCRFs) has further streamlined the process, rendering it more efficient and less susceptible to errors. Moreover, the emphasis on clarity, logical arrangement, and adaptability in CRF design highlights their significance in navigating the complexities of modern clinical trials.
Reflecting on the critical nature of CRFs reveals that their effective implementation is essential for the success of clinical research. As the landscape of medical studies continues to evolve, adopting best practices in CRF design and utilization will not only improve data quality but also contribute to the ethical conduct of research. Stakeholders in the field are urged to prioritize the development and optimization of CRFs, ensuring they remain an integral part of the clinical trial process for the benefit of patient safety and scientific advancement.
What is a Case Report Form (CRF) in clinical trials?
A Case Report Form (CRF) in clinical trials is a specialized document used to collect information from each participating patient, recording their health status, treatment, and outcomes during the study.
Why are CRFs important in clinical trials?
CRFs ensure that information is collected consistently and accurately, which facilitates the analysis and interpretation of results.
How does bioaccess® improve the patient enrollment process in clinical trials?
Bioaccess® allows studies to achieve patient enrollment 50% quicker than conventional Western locations, resulting in substantial savings of $25K for each patient.
What role does FDA-ready information play in the CRF process?
FDA-ready information simplifies the CRF process by ensuring that the data collected meets regulatory standards from the outset, thus eliminating the need for rework and delays.
What are electronic Case Report Forms (eCRFs)?
Electronic Case Report Forms (eCRFs) are digital versions of CRFs that are gaining popularity due to their efficiency and simplicity in managing information.
How can bioaccess® features enhance the research process for startups?
Incorporating bioaccess®'s features can effectively address the frequent recruitment challenges faced by Medtech and biopharma startups, enhancing the overall research process.